Join The Cornucopia Institute as we keep you informed via web updates and live tweets from the National Organic Standards Board (NOSB) meeting online.
We will be sharing the play by play of the meeting on April 28, 29, and 30 below and with our Twitter followers at #NOSB or by simply following our stream.
For background on issues up for discussion at the meeting, see:
- Cornucopia’s formal written comments.
- See Cornucopia’s formal written comments on the Native Ecosystems issue.
Read Cornucopia’s notes on the NOSB meeting beginning April 28. Stay tuned for reporting of the April 20 & 22 public comments.
Friday, April 30, 2021: NOSB Meeting
12 PM ET: Crops Subcommittee (CS), Proposals
Crops Subcommittee Rick Greenwood leads the discussion on crops issues.
Paper Pots – proposal


Steve Ela summarizes the comments on this proposal: Most people are in favor of us passing this. A number of technical comments:
- In terms defined, would like addition of words “typically in paper.” This is a very wide definition; there can be wholly synthetic papers on the market, as well.
- The listing is missing a requirement for continuous improvement, which would also possibly include commercial availability if a higher biobased content product was on the market. We have talked about this before. Difficult to get that wording in that was satisfactory to people. The Board on the whole does want this to be a continuous improvement and as more biobased products become available, I believe that listing will be reevaluated.
- One of the suggestions was using the seed standard – one of the issues is that papers themselves are synthetic and difficult to separate out biobased vs. non–biobased. Tough to do with some of those things because biobased does not necessarily indicated biodegradability and vice versa.
- Add language from last time regarding other 40% beyond the 60% – and we added some language on that. It is by weight.
- Another comment was to not allow virgin paper. Using recycled paper is very difficult because of the variance of the quality. There is a really a very little amount of virgin paper used in these pots.
- Some people asked that we provide standards by which biobased content would be evaluated by qualified personnel. By doing so, we created another set of comments regarding who those “qualified personnel” are – that’s a tough on. One person said they would like to see manufacturers listed by an MRO to provide a consistent product between certifiers.
- OMRI noted they would like the definition to include language from the…?
- We start to chase our tail a bit – in the original proposal we did not include any language on nutrients or pesticides – and we were asked to add language saying that these materials would have to be on the National List, and now people are asking for this wording to come out.
- Inclined to think that we are probably at about the right spot in making people uncomfortable on both sides of the aisle and thinking we can leave the wording up to the Program at this point.
- Finally, a number of people suggested listing changes in terms of the paragraph where they are listed. Good comments. I do not think they preclude us passing this, as it is up to the Program to write the rule. In our listing of these things, we are trying to help the program in saying where we thought they should be, but this is probably not a reason to not accept this proposal.
Stive Ela: Most comments were positive in favor of passing this. Question & comments?
Sue Baird: I appreciate all of the work on this. I think this needs to go forward.
Logan Petrey: Curious if this bleeds into plant tape?
Steve Ela: Intent is for it to cover this. We have not heard much from seed tape manufacturers, but we are thinking this listing would include those.
Wood Turner: I appreciate you acknowledging the issue on virgin paper. I continue to believe that the beauty of organic is that we are not contributing to other issues in the world, so I do get concerned that there is not enough innovation happening to move away from virgin paper, or that we are not limiting the source of the virgin paper, if it is used. This is an issue that is nagging at me.
Steve Ela: At least on manufacturer has testified that they are using a sustainably sourced paper. As a company, they are taking a stance on that. I cannot speak for other manufacturers. I agree with your comment. The scale does make a difference, but I’m hoping that manufacturers will take this seriously. Could not figure out how to word that in, but hoping this will get reviewed at the next sunset regarding the annotation needing updated in terms of ingredients and biobased content.
Amy Bruch: The next item that we are discussing – Bio-Based Mulch Film (BBMF) – has the built-in continuous improvement clause into it. It almost seems as if it is easier to do all of this all at once than to try to 5 years from now get a work agenda item to work on further annotations when hopefully in 1-2 years there will be improvements to hold folks to a higher standard. Is it worth it to include that in this clause now?
Steve Ela: We initially did include a biodegradability clause, and while some people want that noted, we had a number of people ask us to take it out. The wording becomes very difficult. We did have something similar toward the 100% biobased, but you can be 100% biobased and not be biodegradable. The definition of the commercial availability in this case of what items changed – cellulose content, biobased – we did not want to shoot ourselves in the foot of requiring some increase in something but having a worse product. On the BBMF, we are seeing some of those comments – 100% – what does that mean? Do you have to use it? What if there are better products that are 99%? As much as I don’t like leaving it to future Boards without having it written in, the devil in the details on this one. I hate to say it, but we are kicking the can down the road.
Kyla Smith: Thanks CS for your perseverance on this. Certifiers are grateful for the process that the Board and program went through to continue to allow these while we worked on this.
Steve Ela: In the cover sheet to the program, if it passes, I will note some of the concerns that were brought to us. The stakeholder input has been really valuable – want to thank them for all of their thoughts on things that we didn’t think about.
Amy Bruch: There was a commenter – certifying group – that had concerns and questions about annotating this in 205.2, since this is specifically more of a category and not a generic substance like most of 205.2. Overall, there was consensus in adding this to 205.601, but wanted to bring this concern up regarding 205.2.
Steve Ela: In talking with the program, the listing at 205.601 is for Crop Planting Aids, but we needed at definition of Crop Planting Aids. Some of these “where they go” issues can be addressed by the Program on how it makes sense in the regulations.
Kyla Smith: There is some precedent for this type of framework with a definition and placement – BBMF. ACA does have a continuous working group regarding materials. If there are questions, I think that group could take up getting collectively aligned with questions or interpretations of that.
Classification Motion: Motion to classify paper-based crop planting aid as a synthetic substance.
- Vote: Yes – 14; No – 0; Absent – 0; Abstain – 0 . Motion passes.
Motion to add to 205.2 Terms Defined: Paper-based crop planting aid. A material that is comprised of at least 60% cellulose-based fiber by weight, including, but not limited to, pots, seed tape, and collars that are placed in or on the soil and later incorporated into the soil, excluding biodegradable mulch film. Up to 40% of the ingredients can be non-synthetic, other permitted synthetic ingredients at §205.601(j), or synthetic strengthening fibers, adhesives, or resins. Contains no less than 80% biobased content as verified by a qualified third-party assessment (e.g. laboratory test using ASTM D6866 or composition review by qualified personnel). Added nutrients must comply with §205.105, 205.203, and 205.206.
- Vote: Yes – 14; No – 0; Absent – 0; Abstain – 0. Motion passes.
Motion to add to 205.601 (p) Production Aids: Paper-based crop planting aids as defined in 205.2. Virgin or recycled paper without glossy paper or colored inks.
- Vote: Yes – 14; No – 0; Absent – 0; Abstain – 0. Motion passes.
Biodegradable biobased mulch (BBMF) annotation change – 205.601


Asa Bradman: Issue is the use of BBMF – important to use the word “plastic” here, although some people do not like that – we are really talking about plastic films. Plastic can be made from petroleum sources, but also biobased sources, as well. To frame the issue: we have a current listing for BBMF – the 2 key requirements is that they not be produced with excluded methods and 2 – that they be 10% biobased. There is concern about traditional polyethylene (PE) films. Because there are no 100% biobased films available – plant-based carbon sources for the polymer of the film – and there are real philosophical concerns about the use of petroleum–based inputs for organic agriculture. There is a lot of support across many sectors to change the annotation regarding requirement of 100% biobased.
I think there is some real benefits to have an alternative to standard plastics. Is it reasonable to look at this as an alternative to plastic films? Is it reasonable to do a comparative risk assessment for this material against PE films? I think the answer is yes, but that is debatable. If we are doing a real risk assessment, then we need standards.
Comment summary by Asa Bradman:
Some folks commented that they hate using plastic mulch… We used to take our used plastic mulch to be recycled, but now we cannot. Plastic mulch is absolutely necessary for organic production; take it away and prices will soar and production will plummet.
A lot of depth for support for considering something that is less than 100% biobased. There is also support for the idea of an incremental approach. We made an aspiration change to allow 20% non-biobased. How did we come to that? As far as we know, there are not products out there that are 80% biobased and 20% petroleum based. In some ways, we borrowed that from the paper production aid rule. There is a certain logic to extend that to other materials. Some manufacturers said they could potentially achieve an 80% biobased product.
Other concerns about the language so far – I do not think we got all of the wording right, and I do not think we got the notion of continuous improvement right. I think we need more discussion on that – might send back to committee to try to get the wording right.
Verification is a challenge with the current wording. Suggestion that we look carefully at the bio–preferred products. There might be issues with having them made with excluded methods – but I think we need to look at that more closely.
I appreciate all of those who feel this material is not ready for primetime, and that we need more research that we are going to be introducing a petroleum material directly to the soil. I made the point that we already allow a component of petroleum to be used as a pest control in horticultural oils. One of the most heavily used pesticides. We already have a precedent that we are allowing petroleum to go into the soil in agricultural environments.
Comments that this was undoing the work of 2012 when the biobased standard was first made. This gets back to relying on precedent and when do we make a change? We are 9 years later, and circumstances have changed, and plastic use in organic ag has exploded, and that’s something we should all be thinking about. I think that some people are actually doing container growing by wrapping the soil in plastic. Even with PE films, there can be plastics that are left in the soil and probably microplastics that can be leaching in to the soil. When we talk about “plasticulture,” I think it is both potentially in container settings and soil settings. The term actually comes from our strawberry production systems where we are basically mounding the soil into plastic covered rows.
To emphasize, as laid out in the proposal, we cover both the range of reason to allow this and range not to allow this. There is a depth of support in many sectors. I do think we should consider sending this back to subcommittee to see if we could improve the language.
Brian Caldwell: I’m conflicted on this. I can see both sides with really strong arguments. What is tipping me in favor of this right now is that 2 different research groups that the mulches that are available now, some of them are 100% biodegradable. That really makes a big different for me. Over the years, I’ve spoken to several small-scale vegetable growers who have said they were not organic specifically because they could not use BBMF and refuse to use PE mulch.
Logan Petrey: You mentioned a lot of people talking about taking plastic to the landfills, and that gives a bad picture. You mentioned later that there are plastics left. I have actually had to rip up plastic, and it does not come up like a bed sheet, and there is a lot left in the field. I can see where using the traditional plastic can be limiting based on crop rotation and full utilization on the crop program. I am excited about this. I have seen it in use and was impressed with how quickly they went away.
Wood Turner: How do you reconcile these parallel paths between paper–based planting aids and BBMF and the very real reality that paper pots are also going to have similar components as approved that will be contributing similar synthetics to the soil in the same way that this annotation would in the case of mulches – how do you reconcile the feedback from the community on those two different materials? Should we be thinking about them in the same context, or are they two different things?
Asa Bradman: Part of me feels like if you support the paper pots rule as it is written, you have to support this, even though we are talking about a largely different scale. The Paper Pot rule leaves the 20% open to be nylon fibers, which are not biodegradable. A difference with the BBMF is that they have been designed so that bacteria can munch on them and they should decompose. This material should be less persistent and impactful than the paper pots. There seems to be a real need in the community for this material. It is hard to reconcile them. There are very thoughtful comments in the public comment regarding do we want these plastic mulches at all? I do agree that mulching with natural materials and that’s what I do in my garden. But the reality is that plastic has become entrenched in organic, and it seems to me that this product could be an alternative. I hope that they are 100% biodegradable and that we wouldn’t be introducing another source of microplastics into the environment, but we are already doing that.
Kyla Smith: As a certifier, I think I tend to hone in how we are going to enforce this. One commenter was talking about that. It is really the consistency of how the terms are being used. Right now, the term in 205.2 is “BBMF”, and then we start to introduce a new term with the word plastic in there. It is not because plastic is in there, but it is because it is now used inconsistently. I would encourage us to use to pick a term and go with it. If we want to replace BBMF with BBPlasticMF, then we need to do that and stick with it. Also, what I picked up about the percentage is the aspirational approach, and I understand trying to align that with the paper-based planting aids. When I was looking at the background information and all of the resources that we have to look at, I’ve noticed an uptake in that %. In the 2016 materials, I saw a 10-15% biobased, and now it is 60%, so there is that increase. However, it is still aspiration, and we will not be able to have products that meet that. We do not know when we will be able to have a viable product on the market. I think we should put a % in that could be achievable today or delist it. Then my last comment is about the term “availability.” Want to make it clear so that certifiers are able to enforce the annotation.
Asa Bradman: Definitely some issues with the wording that could need some fine-tuning in subcommittee. In terms of the issue of 80% rather than using currently available materials, I took my cue from the paper pots as a threshold. That might leave the frustration out there that we are still not allowing the product to be used, but as you have noted, there has been increase in the bio-based content. This sets a higher threshold, but I understand that it is potentially achievable.
Amy Bruch: The current situation with PE film is far from ideal. Is the alternative to PE film building a better film, or is it addressing how to improve the entire situation. The volume of the amount of plastic that is being used is pretty concerning, and that is how I can differentiate in my head paper pots vs. This material. The multiple use year after year is concerning. I’m concerned what it looks like after you have base-loaded system year after year. Most of the microbial activity in that top 4” of soil – if you want these plastics to degrade, it will have to be in those top 4”. I think more research needs to be done so that we get this right when we make this annotation change.
Asa Bradman: I hear you. We could look at the rule and if we had 90% biodegradation after two years, it wouldn’t be that hard to model plastic buildup in different soil types. That might be something to do to inform that discussion.
Carolyn Dimitri: My question comes from my training as an economist – what triggers farms to undertake specific materials? Do we have an understanding that farmers are actually going to switch to this? Or is this going to encourage people who are using something else to switch to this so that you are adding more plastic to the soil over the long-term to this product. I would like to understand how this would change farming practices? Are we going to prohibit plastic sheets from being put on farms?
Asa Bradman: I don’t know if this is a better system than PE film – I could vote either way or abstain. I do not think it is actually worse than PE films. There is support for this in the community across broad sectors. If I were voting about PE films right now, I might vote no to relisting, and that would be a huge disruption to the market. I wish we were using plasticized materials – I think it is a bit of a stain on organic. It is used across all sectors – big and small, corporate and not corporate, Real Organic Project (ROP) or not – it is everywhere. I think there are real philosophical issues with this one.
Wood Turner: I fundamentally agree that organic should be a real contributor to a more circular economy. It occurs to me when listening to this conversation, we have fairly tightly defined the research priority to this. What we are really talking about here, it shouldn’t be that narrow. Frankly, we need to understand ore clearly the life-cycle impact of plastics on soil – period – the end. It should be about the true life-cycle implications of the use of plastics. I am going to argue from a research priorities position that we expand the view of what we are really asking for in the way of research to go well beyond BBMF.
Kyla Smith: Was going to try to answer Carolyn. More from a not sure about those that are currently using plastic mulch and if they would make that switch – probably some would and some wouldn’t. I do think we will get more operations go to certified organic if they are allowed to use these materials. I guess that’s a philosophical question of whether or not we want those operations under the tent.
Mindee Jeffrey: I am emotional about this issue. At Good Earth about 15 years ago, we did a big push on how people could stop choosing plastic in the store. People want to be real contributors to positive environmental change. Organic leaving microplastics in the soil, I cannot get over it. I do not enjoy disagreeing with Asa. I could be comfortable sending this back to subcommittee and see if more agreement comes from stakeholders after we do that work, but I’m not there yet. Looking at the ethos of organic and the scale at which we have succeeded means to me that I do not have to compromise more on industrial scale.
Asa Bradman: I appreciate that. For me, it is the idea of a petroleum product. But there is this comparative risk assessment idea – plastic use is exploding and increasing overall – look at strawberries in CA, other berries, I’ve seen it in tomatoes – my understanding that plastic use in organic is exploding, and those are introducing microplastics into the soil and environment in general. Is this a better system? If it fully decomposes, we would not be introducing microplastics into the soil.
Carolyn Dimitri: Following Mindee’s line of argument, I think another important research question is to try to understand what are the implications for overall plastic use for farmers if this type of product was allowed. More of a social science research.
Nate Powell-Palm: I hear Mindee clearly and the idea of strawberry growers innovating clam shells with papers. I think that we can figure things out in organic, and sending this back to subcommittee is the way to go. This is important enough.
Steve Ela: I was the descending vote out of subcommittee not because I disagree with Asa at all. I am very much against PE mulch – it drives me crazy – and I really struggle with what the next step is. I am not convinced at this point that the BBMF is truly biodegradable in most systems. I’m glad Asa decided to bring a proposal to this meeting to frame the issue and really helps people to respond to things and try to narrow it down.
Motion made that we go back to subcommittee: Mindee Jeffery, seconded by Nate Powell-Palm.
- Vote: Yes – 14; No – 0; Absent – 0; Abstain – 0 . Motion passes.
1:20 PM ET: Crops Subcommittee (CS), Sunset Review
Ozone gas

Rick Greenwood: Ozone works by oxidizing plant tissues similar to chlorine, used as a antimicrobial agent. Overwhelming support in comments. Advantage over chlorine – no residue left. Used in municipal waters. Relationship with atmosphere worries people, but used in a closed system so no off gassing. Potential for injury in use, but doesn’t seem to be a problem because of the way it’s used. No comments against it.
Kyla Smith: Often used as a fruit sanitizer.
Rick Greenwood: Seems to be fairly effective when used in closed systems. Didn’t see anything in particular about fruit in comments.
Steve Ela: Have heard of it used in irrigation lines, but can’t speak personally. It would be an important use in cases where water was directly applied. It used in dump tanks in packing sheds sometimes.
Paracetic acid

Wood Turner: There’s a lot of use in the community. Favored by many because it is one of the most effective materials in disinfectants that does not involve chlorine. Across the community, extraordinary amount of support. Falls in conversation about sanitizers and what provides the most efficacy. The use in a crop setting is different in a facility because there’s not the same considerations for outdoors. Most of the international community supports it. Very widely used. Some irritation when substance is mis–used – but not a major concern in the community. Some concerns around the source, but overwhelming support overall. Running list of stakeholders who want to see it listed.
No questions.
EPA List 3 – inerts of unknown toxicity

Note: allowed use is only in passive pheromone dispensers.
Steve Ela: List 3’s play an important role concerning pests that effect many crops. Used in many applications – apples and peaches. Only way to control organically in these cases. All arguments made for list 4 can be made for list 3. Not applied to the fruit, contained in the dispensers. Lower amounts are used and not directly applied. Biggest thing is they are on a defunct list – there is lots of comments from stakeholders. One was recommended to be taken off the list – number of stakeholders commented that this was not a proper listing. We can encourage or ask in stronger language that these have to be dealt with, they can be dealt with list 4 ingredients. At this point there are no alternatives. Do passive dispensers diffuse in the environment? They do not because they are much heavier. If removed, would cause significant disruption in the industry. Very widely used in organic and conventional. Biggest recommendation is to change classification, individually instead of in a large group.
Asa Bradman: A little frustration that we have not dealt with list 3 and 4 issues, when we could have dealt with them a long time ago. Could have dealt with them individually instead of in lists. Can avoid drama in the future.
Steve Ela: That comment is shared widely by stakeholders. Jenny said they have released notice of rulemaking to create movement on the issue. To stakeholders: I hope that solutions are put into those comments and not just concerns. It’s up to the stakeholders to give ideas to the program. If not, will continue to be a quandary.
Jenny Tucker: Wanted to clarify that I have committed to moving this forward. Have drafted a notice for rulemaking but it has not been published. This is a process that worked – community came together on comments and agreed that it was important. System works for these priorities.
Steve Ela: I appreciate you letting us know it is in process. Recommend to stakeholders they provide suggestions in comments.
Kyla Smith: A question for Jenny based on her comments. Clarification on whether the rulemaking is on the agenda for spring.
Jenny Tucker: Unsure. Definitely on the fall agenda. Once we write it it has to go through clearance. IT is way further in the pipeline compared to April. It is on a list called the critical priorities lists. No specified exact deadline. We’ve made progress since committing in the spring.
Rick Greenwood: One comment. Part that bothers me the most is where we have the comments where it says: inerts of unknown toxicity. It is not a good title to have. We’re so specific on some things, but not on others.
Copper sulfate

Copper Sulfate 205.601(a)(3) – For use as an algicide in aquatic rice systems, is limited to one application per field during any 24-month period. Application rates are limited to those which do not increase baseline soil test values for copper over a timeframe agreed upon by the producer and accredited certifying agent.
Copper Sulfate 205.601(e)(4) – For use as tadpole shrimp control in aquatic rice production, is limited to one application per field during any 24-month period. Application rates are limited to levels which do not increase baseline soil test values for copper over a timeframe agreed upon by the producer and accredited certifying agent.
205.601(i)(3) – (3) Copper sulfate—Substance must be used in a manner that minimizes accumulation of copper in the soil.
Jerry D’Amore: Made by treating copper metal with metal sulphuric acid. In comments several were opposed, given lengthy comments. Most of the comments came from rice producers. Most opposed were focused on current annotation and our process. Needs to be more specific. Need to continuously improve.

Not response to second question indicating that it is a heavy list. I believe not relisting copper sulphate would be damaging to organic rice producers. Recommend relisting copper sulphate. Through this process was very heartened by the tenor of the stakeholders. Neither were entrenched in their position.
Brain Caldwell: The previous slide I saw on my computer screen was a bit different than what I have in my notebook. There is a 3(e) listing in there that I do not think is in my notebook – is that separate than the use in rice production? Since it can be used as both algicide and tadpole shrimp – is that two uses?
Jerry D’Amore: That is part of the feedback that we are getting – that questions like that have not been addressed – and it may allow two applications in two years. That needs to be addressed. I’m seeing 3(e) on this slide for the first time, and I’m not prepared to address that.
Brian Caldwell: I’m with you on that one. Copper Sulfate was part of a really old miticide from way back. I do not know so much about how rice is grown in CA, I assume that the field has to be dry for the rice to be harvested. Is that process where the water seeps into the soil or where a gate is opened and the water runs off? If it is the second, then a lot of copper sulfate could be run off the field into some waterway somewhere.
Jerry D’Amore: There is significant rice production in the US and limited in Canada. I do not know how the field is dried.
Steve Ela: There is a little misunderstanding – in this listing, the second is listed as an insecticide, but for use with tadpole shrimp control. You do not have to address the mite usage.
Brian Caldwell: I’m thinking that the water issue could be addressed by the committee.
Nate Powell-Palm: Jerry, could you speak a bit about the state of continuous improvement for rice farmers. It seems that this material is being used somewhat sparingly, but that rice farmers are on the hunt for a material that could be used in its place. What do you think the state of the industry is, and could we work towards a reasonable path toward de-listing in the next five years?
Jerry D’Amore: I see a community in rice farmers who have been and is looking for alternative solutions. With that said, we have given them this tool since 2011, and once a tool is given, it eventually becomes essential. I would say at this point it is an essential tool for their ability to be in the organic world. I would further say that the notion that we need to show forward process to finding a way to take that substance out of the toolbox is where we are all headed. If I were around in five years, I would be disappointed to see ourselves asking the same questions.
Chlorine materials
Includes: i) Calcium hypochlorite. (ii) Chlorine dioxide. (iii) Hypochlorous acid – generated from electrolyzed water. (iv) Sodium hypochlorite 205.601(a)(2) – For pre-harvest use, residual chlorine levels in the water in direct crop contact or as water from cleaning irrigation systems applied to soil must not exceed the maximum residual disinfectant limit under the Safe Drinking Water Act, except that chlorine products may be used in edible sprout production according to EPA label directions.

Wood Turner: We have talked about these in Handling and Livestock. I believe a comprehensive approach at how we look at sanitizers needs to be contemplated as we move forward. Not a lot more to say about these materials. I think we talked them through quite thoroughly already. We all know that chlorine materials have some human health concerns, concerns about aquatic systems and habitat. There are concerns that we all know and are trying to navigate. The lingering issue for me is whether or not the use of chlorine materials in crops systems for cleaning equipment or irrigation lines or the like is fundamentally the same FSMA consideration that cleaning surfaces in a facility or in livestock facility and whether or not there is a difficult category of uses on crops. I’d like to hear from stakeholders on that.
We’ve also had this very robust conversation about plastic in organic. We all share the concern that petroleum-based product us is sub-optimal, and one might argue the same with chlorine material. I will also acknowledge that Emily Oakley helped to draft some of the framework on how we look at sanitizing materials in general, but the community did not like that I included it in the published materials that went out. I wanted to try to use it as a way to create some kind of continuity between the sanitizer panel that we had in the fall and how we are looking at these materials and potentially a comprehensive review of sanitizers.
Asa Bradman: Thank you, Wood, for your work on this and our discussions on chlorine materials.
Steve Ela: We personally do not use it because we pick dry, but for those folks that have to hydrate because they pick things that are very perishable, I think they would have to use it. There is also a scale issue with some of these sanitizers – larger facilities may be able to use a more diverse group of sanitizers, but smaller groups may have lack of access to different things.
Magnesium oxide

Amy Bruch: Wide range of uses outside of this particular use. This is the first sunset review for this product since it was added to the national list. International list – no references for sue in crop production. Received a handful of stakeholder comments generally in favor. One comment suggested included a time-frame for future review. 5 questions and general answers from commenters:
- It currently is used.
- Alternatives: There are a few alternatives, but they’re not commercially available or do not meet physical specs
- Humates can support biological life in the soil.
- Non-synthetic alternatives? No addressed in comments. Will need to be re–evaluated. Perhaps TR will address this, TR has been requested.
- Mainly in the manufacturing process. Carbon dioxide is released. If not available, humates would need to be released in dry form causing exposure to dust.
No questions form the Board.
Calcium chloride

Logan Petrey: A lot of support from commenters. Used in fruits, vegetables. Blossom rot, bitter pit, cabbage issues, and others. Some are not seen until post-harvest issues which can lead to costly rejections. Second question was about alternatives – no matter how much calcium you can have in the soil, it does not prevent deficiencies. Calcium is a nutrient that is taken up through a transportation rate in plants, and no matter how much you add to the soil, it does not always amend the problem. Once it is in the plant, it is considered immobile. A lot of support from commenters. Widely used. Do not see any negatives. People support the relisting with the annotation.
No questions or comments from the Board.
Rotenone

Rick Greenwood: This is a potent non-synthetic botanical. It is only registered as a piscicide (fish killer)and not available for purchase in the US. Banned in EU and United Kingdom. We had a few comments on it – keep it as a prohibited substance. I do not think there is much else to say about it. It is for sale in some places around the world, so we want to keep it on 602 to not allow it from coming into the country.
No questions or comments from the Board.
2:15 PM ET: Lunch Break
3:00 PM ET: Crops Subcommitte, Petitions / Discussion Documents
Ammonia Extract—petitioned for addition to 7 CFR § 205.602

Steve Ela: Nitrogen is one of the things that is constantly an issue as a grower. It’s a complicated topic. The number of thoughtful comments were appreciated. Asked for references; our stakeholders came through (not read all scientific references yet, but will).
Break down presentation (covers 6 things based on questions asked to stakeholders):
- Professionalism: Not as much professionalism from stakeholders in this topic. “Obvious commercial bias” references by commenters. Might raise questions about the NOSB. Say to stakeholders: offensive to get comments like this; these comments are not appropriate. The NOSB looks at each petition as written; we take all of them seriously.
- Definition of “ammonia extracts”: Does the petition definition fit? We will have struggle coming up with a definition. [Reads petition definition of “ammonia extracts”]. The definition in the petition raised concerns form many stakeholders, most saying definition is too broad; valid criticism. Many ammonia products have been approved for years and we might not want to include those products in the definition. TR/stakeholders have identified two different processes in creating ammonia extracts: ammonia stripping and ammonia concentration (take original material and removes water). When we had oral commentors talking about their products still including soluble organic compounds [carbon] they were speaking about concentration method. Stripping methods take ammonia out of the product so that it is pure ammonia. One includes carbon and one does not—we have to consider how widely we want to cast the net. We may want to limit a certain level of ammonia. OMRI has noticed product review applications increased recently for high nitrogen fertilizers; variations on both types of creation methods. Another note: non–synthetic fertilizers that test above 3% are considered to be higher risk of mis-use in soil contamination; use must be part of OSP. These should not be used in isolation, requirement for soil building/natural resources still needs to be complied with. The scope of the petition does not cover other fertilizers that also contain ammonia (but still contain organic matter, ex. Fish emulsion).
- Soil health and biodiversity: A lot of the comments addressed this issue. Always have to go back to OFPA that gives us the seven criteria we use to evaluate substances. Petition said that if we did not prohibit these substances, we would be going against OFPA. Plants already uptake nitrogen in ammonia/ammonium forms. If we apply manures the process of mineralization occurs throughout the year, so there are some comments that nitrogen can be lost when plants are not using it. Effect of bio-based fertilizers was more evident in low-biological activity than in high-biological activity. Comments promoting ammonia extracts: capture allows precision in apply to fields, limits volatilizing to atmosphere. Reduces chances of buildup of P in soil profile (which can occur from using manures). Potential groundwater contamination from manures and other org N products are applied when the crop does not demand it. Comes down to carbon-nitrogen rations; lower C levels than N there will be reduction of soil C. Materials that are higher in C will increase characteristics related to soil organic matter. Comments also note/remind us that: Prohibition of synthetic high nitrogen fertilizers was a primary motivator of the organic motivator. Products that mimic synthetic nitrogen fertilizers should be considered the same way as inherently incompatible to organic principles. Note that some of these fertilizers are of higher solubility, they are counter to fundamental organic principles. “Law of return” where residues are returned to the soil through tilling, mulching, etc. Most organic practices work on recycling materials into the soil; special attention should be given to SOM. What supports nutrient cycling? Manufacturers comment that these fertilizers are not meant to be the sole source of fertility. Note that: comment that tried to do meta-analysis from these types of fertilizers: crop-rotation, legumes, and other organic inputs all have their own issues that affect soil health and it’s difficult to make over-arching statement for these kinds of products. Previous management history also affects soil organic matter. That’ a high-level summary of soil health and biodiversity issues.
- Yields: Noted by commenters that these extracts can help increase yields to help organic compete in the market. Conversely in the public comments: citation to organic farming trials by Rodale—no statistical difference in yield between conventional and organic. That to me counters argument that there is a need for extracts to increase yields. Long-term consequences of conventional practices harms soil, and exacerbates ongoing environmental risk factors. Organic yields have kept up with conventional yields. Steve Ela comments about organic peaches.
- International recognition: International consumers: sodium nitrate is an issue with Canada; they do not accept crops treated with it! Issue with USA-CA equivalency agreement; needs to be paid attention to. Example: organic tomato growers, may have difficulty exporting bc of use of small amount of sodium nitrate.
- Highly soluble fertilizer sources: General comments note that non-synthetic mimic synthetic fertilizers and have been prohibited in the past. One past NOSB voted to completely prohibit; previously limited to no more than 20% applied. So there is some precedent to limit usage. Carbon value of original source material not contained in product those products have also been prohibited; part of the bio char debate for example. May not comply 2004 NOSB recommendation. Alternatives: there are some fertilizers with over 10% nitrogen content. Commentors note that it is time to regulate highly soluble N; it’s appropriate to limit them. Ammonia extracts are not unique that allow growers to feed their crops w/o feeding the soil. Some commentors wanted all fertilizers that don’t feed the soil to be regulated; organic farmers should be required to add carbon back into the soil.
Steve Ela: Finally, hate to create situation where grower could use 20% of each different highly soluble source (ex. Sodium nitrate, fish, etc.) and put certifier in the difficult position of writing a noncompliance where this is not clear. Tracking compliance in the face of uncertainty is a concern.
Curious to hear from Amy and Logan as other growers. On our farm we try our best to get fertility form vegetative sources with manures as a small addition. Some commentors noted that manures were the main source of N, and that’s why these extracts would be useful (to avoid P build-up).
Amy Bruch: On my farm (row crop, pulses, oil seeds, etc.) we look at fertility 3-5 yr basis. Can’t rely on just manure-based system because of accumulation of P and calcium. Complementary rotations. Robust crop rotations help with fertility, especially with corn, and help with weed management. Programs/rotations are multi-faceted. Total system approach: growing our N is important, helps create biodiversity, increases soil organic matter (SOM) which helps with future N needs. The legumes are good at generating N. Apply feather meal; use some biological activators (humic acid). We take annual soil samples, part of water districts where we have to report N levels to water board. We have Great resources that helps us analyze our soils short-and-long-term. Manure application is not a one-turn benefit, it’s multi-year.
Logan Petrey: I am in the southeast. We have a lot of rain and sandy soil, so we run into leaching issues of N, sulphur, potassium, etc. It makes managing fertility Hard; we often do our crops in the winter to avoid pests. The cold prevents manure-based fertilizers from breaking down, preventing N release. So soluble N are not ideal in an organic system it helps prevent crop failure. Fertility is the #1 tool to fight against insects and foliar pests. Once plant is looking healthy, it’s more robust against disease. Although our program is not built around soluble N fertilizers, we do use them when we need them. It does not reduce our use of crop rotation, which is extensive; it does not limit our use of natural fertilizers. We use a lot of chicken litter (broiler side preferred to avoid phosphorus buildup). Cover crops (mostly in summer) are also used to combat fertility issues. No grower in the area can use only cover crops because of the amount of leaching we get, in part due to planting season (which is during hurricane season). We can’t just do pre-plant application, then do 2-3 side-dressings per crop. Manures are diff to get the amount of N needed; feather meals have high N availability. 2-3 week release of N with each pre-application. But when the crop is 2-3 weeks from harvest you side-dress when needed (to prevent damping off issues). There is loss with natural fertilizer also. Agree that limits on extracts are fine because I prefer to use natural fertilizers. But the soluble fertilizers are needed in this area to maintain yield. Lifetime of FL experience.
Steve Ela: Would like to come to a proposal next fall. Feedback from rest of Board?
Wood Turner: Trying to wrap my head around this. One question: not comfortable that there is a clear enough definition of what the material is? This petition is a blanket; not sure we are capturing nuance we are hearing in comments and Board. Something lacking that could benefit from a clearer definition.
Steve Ela: we didn’t get a lot of response on the definition issue. Like with BBMF and paper pots, the nuances are important. My bias would be to write a definition that is conservative; we don’t want to take out products that have long been a staple of organic growers’ toolbox. The working in the petition definition might be overly broad. Concentration versus stripping: tend to lean toward preventing stripping because it’s much more similar to conventional synthetics. That’s where I would start; will talk to more people over summer.
Kyla Smith: Yes, we need to hone in on definition.
Steve Ela: As certifier, if someone is using highly sol fertilizer, and you are looking at farm system plan how do you suss out the use of a highly sol fertilizer versus something else required in a soil system?
Kyla Smith: I don’t have the regulations memorized.
Mindee Jeffery: Not an ammonia expert; mulling over highly soluble issue. First reaction we have to prohibit this. If we can get a clear definition I think it would support innovation, especially where we can support limited need of this product. Great definitions and narrow annotation would be great. Is that within the scope of the petition re-write?
Steve Ela: Yes, we’ve done that in other cases. We can say: “We agree with petition, EXCEPT…” is possible.
Amy Bruch: Looking at conversation on BBMF; seems to be need for extracts that are using plastic mulches. Comprehensive systems approach is hard to do with plastic laying on soil. Whole organic approach—we need to step back and look at dominoes of what you’re doing. That concerns me (not having access to soil). As Logan mentioned, we also do split applications. It’s hard to get multi-nutritional sources to your plants.
Steve Ela: Healthy soil means our trees get to eat the smorgasbord.
Carolyn Dimitri: Last two comment follow up: I’m an economist, not an agronomist or farmer. My default is to the organic ethos. It would be helpful: is there a way to have 3 sentences in English that would help consumers understand where we end up on this. Need to distill this so that someone can feel comfortable. That’s my request; I don’t have an opinion.
Steve Ela: We do have to frame things correctly (ex. Organic pet food).
Nate Powell-Palm: I don’t have a lot of experience in southeast, more so CA to PA. When speaking to organic Agriculture’s contribution to climate change; we don’t use ammonia, organic is not super input-heavy. Prices for grains flux a lot. Market stability is due to needing to rotate crops (people can’t grow corn every year). Worst fear is that someone certifier shops and just raises corn on corn because its do-able (with ammonia). Ammonia extracts flies in face of organic ethos. Think that regulating it really hopeful, because of isotope testing. We can make sure people are not just using conventional ammonia. There is an organic place to integrate livestock; don’t have them divorced, ending manure into waterways, and crops having a deficit of soil micro–bioactivity. Think organic crops need livestock nearby, or getting manure onto their ground. Spoke to farmers who don’t have livestock and they do lots of crop rotation (rye for seed, hay, etc.) because they can’t push their system just to maximize corn. Good to communicate to public about organic contribution to climate change.
Kyla Smith: Add 205.203 is the soil fertility standard; we would look at input use and that practice standard as well [as certifier]. Unless material has specific annotation, we don’t necessarily look at inputs outside the wider context of use. It’s not a hierarchical regulation like in other areas of the regulation.
Steve Ela: Fraud – Sample at manufacturer and at farm have to be consistent. You can’t just look at the soil, need to look backwards. Grower oversight red flags is important. Fraud may not be as big of an issue as overall fraud is.
Kim Bruch: OMRI written comments did mention that isotope ratio testing as they had previously commented that it’s not reliable. Something to note. Organic space has debates around fraud in one context or another. How do you manage other than a mass balance? Need to consider as we look at this more.
Steve Ela: Should have probably spent more time on fraud in future write-ups I will. As we work to write a proposal CS will reach out to others on the Board for input.
Kasugamycin—petitioned for use as an approved active ingredient in organic crop production at 7 CFR § 205.601.

Rick Greenwood: A long time clinical microbiologist and lab director – the thing I have seen in microbiology is antimicrobial resistance. Nothing is really sadder than isolating a person that has tuberculosis and finding that they have a very resistant form of tuberculosis. The other is MRSA – incredibly resistant microorganism. The reason these are around is the over use of antibiotics – not only in humans, but also in the whole animal industry. Microbiologists have recognized it, and the bacteria and microbes have recognized it even faster.
This is another aminoglycoside they want to use for fire blight. Comments from growers is that they want another tool in the toolbox. The public and many of the other groups – 250 comments – keep antibiotics out of organic. I think it is a pretty clear message that people do not want them there. Some of the concerns we have – came up in the TR – resistance gets built to these very quickly. Other issue is that when it gets sprayed on trees, animals can get to it.
There are cultural methods to make it more difficult for fire blight – you can control it – it is much more difficult, and there are regional differences. Some areas, not much of a problem. When it has been used, it has developed even in Japan starting in 1965, resistance developed very quickly and could not be used. Also, in Florida on tomatoes. There is history for these things.
Happy to talk about this. I would say our stakeholders really do not want us to have something else come into the production area as an antibiotic. The NOP and NOSB identified several reasons to stop the use of streptomycin and in 2014 stated that the expectation is that antibiotics not be used in organic production. I feel for the growers, but I think it would be the wrong message to send to our consumers. CA has allowed kasugamycin with an application plan, but I think it won’t be long until we see resistance.
Brian Caldwell: As an apple grower, I think farmers have to realize, without our customers, our crops are pretty worthless. There is overwhelming sentiment against adding antibiotics in our production systems. There was quite a fight in 2014 to take antibiotics out of apple and pear production. I’ve been a grower for over 40 years – we do see fire blight, but we do use cultural practices. Most of our varieties have lesser susceptibility, we use resistant root stock, and we do not push our tress – and that’s a big one. A big part of the problem is pushing for production. The market can be strong for all varieties. Less intensive planting systems with root stocks that have resistance – farmers don’t want to do that – but it can be done. And taking it easy on the fertility. That is pretty much my message. We really have to keep in mind that the consumer is the one who buys our products, pays a premium, and supports us.
Sue Baird: In poultry, you have to rotate antibiotics because they build up resistance. If we allow antibiotics back into organic production, they are going to build up resistance. I am thinking about the perception that people are being bombarded with now – that we are diluting the organic standards. If we approve adding antibiotics back into the National List, I think we are going to get slammed hard.
Steve Ela: As an organic grower, fire flight is a big issue for us in pears and susceptible varieties. It’s not a big deal until it is systemic. I think climate change has made it worse. It is really a combination of water and temperature. Water and cool – bacteria won’t expand. Warm and dry – bacteria won’t move. We used streptomycin when it was still on the list. Really useful because you can put it on up to 24 hours of having an infection even – would kick back and help with issues. In Colorado we tended not to use it much, so we did not have resistance. Once those were taken off the list, the next years there were a couple of products released that are more effective. The method now is a systems approach that does involved copper and lime sulfur early in the season. A lot of organic growers use lime sulfur for thinning. The problem now is that we are using a yeast product, but if you are using lime sulfur for thinning, it would kill the yeast, so you have to be very precise in your timing of application. Most of our varieties are resistant, although we do grow some that aren’t because consumers demand them. We have not lost a lot of trees to fire blight, but we have reduced our canopy by 60% in some. I argued against the removal of antibiotics from the National List, but with this material, I would hate to change the precedent set by the Board. I think the resistance is real. My stakeholders would say to absolutely approve this, but I think we also have to vote our conscience and gut feeling. I am going to have a tough time with this one. I’ve never abstained on a vote. This could be one. Leaning that it isn’t acceptable.
Mindee Jeffrey: Not in favor of allowing antibiotics in organic. I think it really behooves us as a community to provide more context to consumers. We have a really beautiful form of democracy, and I think we try to help consumers understand that we review all petitions equally. We will always evaluate each petition neutrally, and then ask consumers to support us.
No other comments from Board members.
4:23 PM ET: Deferred Votes
Ion exchange filtration

Steve Ela: Before we vote, I would like to open back up for a brief comment period from the Board to see if there is anything else that needs to be said before we move to the vote.
Brian Caldwell: I do have some information that was new to me. I was concerned yesterday in our discussion that styrene is considered a probably carcinogen, but polystyrene is not, which is what is listed as one of the materials. And polystyrene sulfonate, which is one of the resin materials, is actually used as a human medicine, and it is 100% excreted. Even if this would get into some products, it sounds like it is not harmful. That put my mind to rest on that issue. I would still like to see this go back to the committee. I think we could vote separately on resin materials. I would also like to hear the group come to some kind of agreement on what it means that the process causes a chemical change in the organic product and what that means in terms of its status. If we do vote and decided not to send it back to subcommittee, I will vote in favor of it, as I think it is more important that the NOP gets some guidance from the NOSB than not.
Asa Bradman makes a motion to send back to subcommittee. Carolyn Dimitri: Seconded. Motion to send back to subcommittee.
- Vote: Yes – 4 – Wood, Asa, Amy, Brian. No – 10 – Rick, Kim, Mindee, Logan, Nate, Kyla, Sue, Jerry, Carolyn, Steve. Motion fails.
Asa Bradman: There would be a cover letter with this, and I think that we talked in the cover letter asking for some specific information – is that correct?
Kyla Smith: I would be in favor of putting in the cover letter that we are looking for specific answers on the legal interpretations on whether or not the ion exchange resins – how their definition within FDA in regarding to food contact or direct food additives align with food processing aids.
Amy Bruch: After the cover letter and that gets answers, does it return to subcommittee?
Steve Ela: I think the intent is to give some information back to the Board, and after that I think it depends what the NOSB and the Program would like. I think that bridge is crossed down the road.
Motion to accept the proposal on ion exchange materials.
- Vote: Yes – 14; No – 0; Absent – 0; Abstain – 0 . Motion succeeds.
4:35 PM ET: Other Business & Closing Remarks
Continuous Improvement and Accountability Act
Steve Ela: A bill that was introduced this morning about Continuous Improvement, noting that USDA has failed to implement and has run into issues with key organic standards (like OOL, OLPP, greenhouse). I’m excited about this bill. The Continuous Improvement and Accountability Act will require the USDA to put together an action plan on past NOSB recommendations.
NOSB agenda review
Steve Ela reviews adgenda items for the Fall 2021 NOSB meeting.
4:45 PM ET: NOSB meeting adjourns
Thursday, April 29, 2021: NOSB Meeting
12 PM ET: Call to Order, Materials Subcommitte (MS)
Steve Ela confirms that all 14 members of the NOSB are present at the beginning of the call.
The Materials Subcommittee (MS): 2021 Research Priorities
Wood Turner summarizes the issues, public comments: Appreciated the NIFA presentation yesterday; it helped clarify the evolution of research priorities and the thinking involved after the NOSB submits them.
Research priorities include the use of methionine in poultry, breeding programs for animals well-adapted to living outdoors, carbon sequestration, and more:

Some comments have indicated a need for research on heavy metals in baby food, clarity regarding sanitizers, and other issues.
NOSB has used draft guidance as part of the Sunset process, and community feedback suggests this is inappropriate and should be discussed more widely.
Sue Baird: Some comments suggested we remove some of the research priorities. NIFA presentation suggested we leave these items on the list. The efficacy of natural parasiticides and methodologies was added for poultry, as fenbendazole was not allowed on the National List.
Brian Caldwell: Amazed we’re still asking for research on parasiticides. Was involved when NOFA-NY first wrote standards in 1998, and they were asking for this information then. Surprised more is not known about this now. Wonder if we could consolidate research to make it easier to find.
NOTE: For Cornucopia’s thoughts on both the parasiticide Fenbendazole and methionine, check out our 2020 public comments. More information on organic poultry can also be found on our website.
Rick Greenwood: Re: human capital – We really don’t know what happens once these research priorities are added; have they been resolved? Never previously understood the link between this list of priorities and NIFA. It is very helpful to know.
Kyla Smith: Notes that an important link is missing in terms of getting the research to the people who need it. How can we get the finished research to the farmers, consultants, and others?
Amy Bruch: I know these research priorities are relevant, but some of these are in our current work agenda. Despite the request to not prioritize the list, we do have clear priorities.
Excluded methods (EM)
Mindee Jeffery: As a board member, tried to educate everything the board has said on this issue. This discussion document is meant as a ‘how can we reset ourselves’. Learn how excluded methods are impacting supply chains. Take the public comments and contextualize them. Major reflections from stakeholders can be organized into 5 buckets.
5 general buckets of types of commentary on this topic (including a summary of quotes and comments from stakeholders):
- Commenters request to move forward on 2016 recommendation. All major stakeholders recognize importance on this course of action. [MS discussion documents can be found on this page.]
- Comments from the seed community. Seed use long tethered to excluded methods discussions. Commenters want help to support and bolster organic seed usage in the community (because there is still not enough available seed, some folks may be using seeds produced via excluded methods). MS needs to tackle seed issue in particular as EM issue and standalone issue.
- Response to “To-Be-Determined” (TBD) list (terms that that not been TBD whether they excluded methods category). Difficult to track these issues while also concerned about field sprays. The clear and consistent request to act on the TBD list in the fall meeting with a lot of information on how that will affect farmers. That was a big push from farmers. One quote from a seed producer asked the NOSB to make recommendations clearly. Breeders need to know if and how they can serve the organic community. Good information from a seed group that outlined how our recommendation on this topic will affect farmers.
- Transparency and protection. Comment summary: info about tech form USDA need to be free flowing; updates to regulations need to be timely. Manufacturers have the authority to determine if their product is going to be regulated; this is problematic. Sec. Needs to be held accountable to organic co-existence with markets that allow GE products. The FDA has no mandatory requirements for food requirements and technically has the ability to assess animals.
- Urgency factor: Comments from retailers, etc. that this urgently needs to be resolved and tracked closely. Consumers are passionate about keeping GMOs out of their food, and when they say no GMOs, they mean it. This is the space that they rely on us doing our jobs so that they can eat in a way that they understand.
Mindee Jeffery: This is a big subject. We will have time in subcommittee to go over all of this feedback.
Wood Turner: Giant piece of work. Want to acknowledge your work on this issue. I feel like we’re in good hands.
Brian Caldwell: Amazing job of condensing all of those comments. I appreciate that Jenny Tucker spoke at length a couple of times to us about excluded methods. One of her points was that the existing definition under the regulations really does cover a lot of ground with EM. Maybe trying to capture all of the new forms of GM that may come out and gene editing and everything like that isn’t as important as enforcing what is already known. Maybe what we need to work on is a mechanism for exposure – thinking about seed varieties mostly, but maybe can extend it to other things in process. The TBD list that we have is about cell fusion, but it is actually specifically named in the definition of EM that it is excluded already (so I’m not sure why it is TBD). Question of how to retrieve varieties that have been in use for a long time; may have to realize that we live in an imperfect world. Demeter has never allowed cell fusion (see article from 2008). Maybe enforcement of the rules that are actually in the regulations is a way to start on some of this and then moving forward from there rather than trying to specify all of the methods that are coming along. They should be captured already if we can ask for information in that transparency part that you talk about from the seed companies. It evidently totally works for GMO corn and soybeans. We should be able to do the same thing with other vegetable varieties with EM.
Jerry D’Amore: I would like to acknowledge your great and thoughtful work on this. I believe this is a path we have to go down with vigor.
Kyla Smith: ACA Materials Working Group did develop together an affidavit for EM that is mostly used for handling ingredients that does take into account the technologies that have already been voted on by the NOSB and lists them specifically for manufacturers to say that they have or have not been used. I know that is about handling, not seed. Question: Mindee talked about in your first bucket urging the program to move forward with the previous recommendations, and based on Jenny’s presentation on packaging things together, wondering if it would behoove us as a Board so this could all get packaged together into one rulemaking or guidance?
Jenny Tucker: Important conversation and insightful – this will continue to emerge – encourage people to read the regulations so that we can frame this into the “we think this is already prohibited because the regulations say this.” To Brian’s point, we are enforcing the current regulations, which area already quite broad. I think it is a very solid umbrella. I think we need to think of how we are framing all of these examples in a way that someone doesn’t say “this isn’t on the list, so it must be allowed.” No, it is a broad definition of what is not allowed here. Need to think about what we most need in a practical way to give certifiers what they need as a practical cool and not open up any loopholes, and then look at how frequently we need to update it.
Kyla Smith: Would it be worth making very clear on our cover sheet what we want the Program to do with this whole package – would that be helpful? I know there hasn’t been a lot of movement in guidance, but as a certifier we really rely on guidance.
Jenny Tucker: This is not an esoteric discussion. These are decisions that certifiers need to make every day. Connecting that to what certifiers need to do is so important in this dialogue for consumer confidence. Really important conversation. I want to help move this along. Let’s remember how strong our existing regulations are rather than have people question it. They are strong. These are prohibited. These are excluded. Let’s always put that first.
Nate Powell-Palm: In tacking on to Kyla’s point – it is not boring at all how we figure out the nuts and bolts and minutiae of how we do this. I want to upvote what Kyla said about wanting those guidances and moving forward with past recommendations on this topic.
Mindee Jeffery: I think part of the pressure comes from the overwhelming development of biotech.
Steve Ela: Just like BBMF and the arguments of where we said, “Not derived from excluded methods,” and I think that to Jenny’s point that is true, but we also see in the fermentation process that creeping in…and we keep bringing up that if the product doesn’t contain excluded methods, is it okay? I would personally say it is not if it was made with EM – but the question is how far back we go. We need to not just focus on definitions, but larger filters that we can run things through to determine if it is or it isn’t a EM. I think it is going to be tough going to keep the prohibition a prohibition without something sneaking in. So glad you are working on it.
12:45 PM ET: Compliance, Accreditation & Certification Subcommittee (CACS)
Discussion Document: Human Capitol Management
Proposal details for this discussion can be found here (this includes the contents of the slides).
Nate Powell–Palm: Kick this section off with history of myself. For ten years, I’ve travelled and worked for a lot of different organic companies. Initially had decided to take an inspector course to get better at paperwork and his own inspections. After taking course, realized there was this underground world that made all of the work possible. When Jenny came out w memo on human capital, whole community said this was something that needed to be addressed. We’ve gone from state to state to $60 billion industry + need qualified professional certifiers to protect integrity of organic. Shout out to Jenny for recognizing important work of inspectors while going forward w resilience in mind.
When the memo came out, a lot of people kicked into high gear. A lot of folks like Oregon Tilth wants to change how things are done thanks to memo. Commend the leaderships in acknowledging the deficits in the profession.
Sue Baird: This has been a great project. Thanks to Jenny and the staff. Wanted to highlight a few things from the human capital document. Asked questions to inspectors: what compelled you to become an inspector? What compelled you to stay in the profession? If you left, why? What are challenges you’ve ID’d as an inspector or to becoming an inspector. Common points we heard: cost + travel time. Positive and negatives on the ability to travel. Professionalism – it appears that some of our inspectors have experienced a lack of recognition that they are professionals. What causes this? 3rd point: compensation for experience. How do we build professional experience as inspectors? What was the environmental impact of travel, how does the job impact personal life? Lack of consistency for certifiers when it comes to insurance etc., which has led to certifier shopping among inspectors.
Responses were overwhelming from individual certifiers, certifier entities, and from IOIA. Commend putting out the survey to certifiers.
There was interesting challenges – how do we go about addressing? There is a lack in the inspector pool. Experienced people in the industry do not want to travel or lost family time. Family is a major consideration for inspectors – a lot of time spent away form family. Providing insurance is a huge issue.
First of all, was perhaps we need to implement a risk-analysis of those certified entities that have more of a risk factor and focus our move in depth inspections for those entities and save the lesser risk entities for a lesser risk inspection. We would have to identify parameters of high risk.
Second strategy identified: How do we apprentice these new inspectors so that the concept of professionalism is ingrained? How do we ensure our industry that the integrity of our products is being maintained?
Kyla Smith: Some things that I saw in the public comment are, as in the proposal, the way that the proposal was structured – there could be entities doing pieces and parts of this – an apprenticeship program, career development, training, etc. Some other commenters talked about a more unified approach through a credentialing program – take those elements and wrap them together into one thing so it’s not so parsed out. Other thoughts from Board members if one strategy is better than the other? Is there an appetite from the industry to have a more centralized certification program – cover qualifications and tiering of entry level, mid-level, expert that would have some pay and other qualifications that go along with it.
Nate Powell-Palm: Wanted to jump on that. When we look at the acute bottlenecks – the apprenticeship program – and how we get that practical training to inspectors, I have always admired how close to the community and grassroots IOIA has been able to remain. Coming up with an entity that is closely related and equally grassroots – such as what Angela & Garth Kahl mentioned – would make it so that we have a fundamentally acknowledged standard of training but also able to fill that bottleneck.
Sue Baird: I agree. I did hear comments that a lot of the respondents would like to see us somehow aligned with secondary education. Some respondents noted they would like to get CEUs that they could then transfer to other things. Interesting and has a lot of validity. If you are going to spend $5,000 for training, it would be nice to get some college credits for that.
Nate Powell-Palm: I couldn’t agree more. If I were to paint the world I would like to live in, I would see IOIA working with a university to make a standardized 2-3 credit course. As a training for IOIA, I could definitely fill 45 hours of lecture time with all of the minutiae that goes into being a good, observant inspector. I think marrying this with a university for a more formalized education component would work really well. I do really like how IOIA has been able to maintain their relationship with the inspectors who are actively working on the ground. I think there is even more potential with the university component, as we would be better able to target schools that are not in the organic realm right now. I think that would be a way to tap into broadening the organic tent, as well. I would tap onto that that personally, I thought it was a real steal to be able to spend $5,000 to jump into a career that would pay me more than anything I could get into from my undergrad degree, which was more than $5,000.
Amy Bruch: So important with the impending SOE, with more strain on certifiers and inspectors, getting them the training now is more critical than ever.
Steve Ela: In this training, if we could help ensure that we have diversity; that we not just fall into the trap of the normal “look” of inspectors. Black colleges and such that we could reach out to to ensure that our inspectors represent a wide diversity.
Motion to vote on forwarding the proposal on Human Capital by Nate Powell-Palm. Second: Mindee Jeffery .
- Vote: Yes – 14; No – 0; Absent – 0; Abstain – 0. Motion Passes.
Discussion Document: Supporting the Work of NOSB

Nate Powell-Palm: As a first-generation farmer, I realize what a stretch it is for volunteer work. And that is true of a lot of people, and we miss out on a lot of great talent because of that. Thinking about how to make this more accessible to more people, this discussion document is a first stab in trying to figure that out. What can we do to make it so that the lift isn’t so insurmountable?
Steve Ela: I appreciate both the CACS and the Program bringing this up. There were a number of comments that we should open an online docket between NOSB meetings for a more transparent information sharing and help NOSB members with information processes. Although we did not manage it this time around, in general we do open the docket between meetings, but that is largely unused – by both stakeholders and the Board. It is always difficult for the Board and subcommittees because our turnaround time is so tight. The time for a lot of exchange is limited, but could be used more so that proposals reflect stakeholder comments.
We also know that there is a lot of concern about undue influence from the Program on the Board, and it was really heartening that all of our stakeholders were in favor of this. Comments were great that worries that the Program having too much control could be allayed. Number of commenters noted they would like the Board members to have some autonomy in making decisions on what kind of help they received. Thoughts were really of providing NOSB members with more technological support, where someone could really provide some help. We already have the TRs, which are hopefully able to provide this, but they have a fairly long turnaround time and are expensive. Looking to have something more readily accessible to the Board. Another recommendation was to have some help around regulatory language around what our intent is so that the Program does not have to interpret in the regulatory language what we meant.
There were some comments that the NOSB members should author their own Discussion Documents and Petitions, as that is the intent of being on the NOSB. Others noted that it would not be appropriate to have outside people summarize or abbreviate the public comments, but that the Board should reading them.
I was pleased by the support and encouragement for us. I know serving on the Board does take a lot of time and may prohibit individuals and those from small businesses to participate.
Any thoughts?
Asa Bradman: Michelle clarified for me that as part of OFPA that participation on the Board is without compensation. Want to emphasize that it also limits people, as well. Not just time, but also money. When we think about diversity…of course we cannot contravene OFPA and that specific designation, but I think that as we think about resources, that could make the difference on that.
Nate Powell-Palm: Thank you, Jenny, for getting this conversation started. Looking forward to moving forward.
1:25 PM ET: Handling Subcommittee (HS)
Jerry D’Amore, Subcommittee Chair leads the HS discussion.
Proposal: Ion Exchange Filtration (IEF)

Steve Ela: Ion exchange was sent from the Program to give recommendation about how ion exchange materials should be handled/certified. This report is updated from the more concrete proposal in the fall that was voted down.
As a high-level reminder: there are many different aspects of em that ion exchange involves.
Widespread agreement that recharge materials should be on National List. Resins in the column have ionic points where ions are attached. As you run them through the column, those ions are exchanged. Sodium ion in the resin may be exchanged for something else we don’t want in the food product. Over time, the ions are spent and must be replaced.
The big disagreement is regarding the resins themselves and whether they need to be on the National List. Are they food contact surfaces, such as piping, tables, and o-rings? Or are they secondary food additives that contribute things to the final food product?
Some commenters noted that ion exchange may introduce chemicals into the food, but they do break down, and they should be listed. There is precedent for their listing in boiler water additives, etc.
Other commenters claim the resins do not belong on the National List. Gets into legal issues that may be beyond the resources of the NOSB. Program should get involved.
FDA regulates food contact substances, and they are not intended to have any technical effect in the final product. It can be argued that the resins themselves are not intended to have a technical effect – just the resins attached to them. The resins themselves are “inert.”
It was noted by one commenter in response to the fact that some of these other items do need to be on the National List that there are processes that can change a product that do not need to be on the National List – baking, for example – it could be argued that some things we allow and some things we do not.
It was mentioned in the public comments, and comes back somewhat through the Harvey lawsuit, and in that lawsuit they interpreted ingredients as including processing aids. Processing aids are something that are included in the food for a technical effect. It would seem that would indicate that processing aids do need to be on the National List; however, in 2002, the Program had issued a policy about what “still present in the food” applies to: ”…substances classified by the FDA as food contact substances are not subject to this definition.” FDA’s classification really does affect whether or not these materials need to be on the National List.
To me, it really does hinge on the program does say that a lot does hinge on food contact or secondary food additive.
There is a wide agreement that ion exchange filtration must be reviewed and approved as part of the Organic systems plan (OSP). Handling Subcommittee recommends that ion exchange is allowed provided that the recharge materials are on the National List and the resins are identified by the FDA as a food contact material and disclosed in the OSP. There is disclosure required.
Received a large number of comments on this. Definitely a topic that has been a discrepancy between certifies, and that discrepancy is shown in the stakeholder comments. I personally feel that this proposal should be passed – basically sends it back to the Program to say get the legal opinion – and the legal opinion of whether or not these are food contact surfaces will determine whether or not they need to be on the National List.
Brian Caldwell: Complex issue. I appreciate you going through all of that. 2 questions: (1) It sounds like you felt the comments were pretty unanimous that a substance that goes through ion infiltration is chemically altered – is that considered non-organic then?
Steve Ela: I think that’s above my pay grade. What is added to the product is technically on the National List if the recharge materials have to be on the National List. I do not know that it really affects how we look at this proposal, but it will how certifiers look at that.
Brian Caldwell: Speaking from the consumer point of view, I understand that a lot of this legal wording is about whether or not there are residues in the final product. As a consumer, I am concerned that there may be residues in the final product, especially when I see that styrene may be one of the products (a cancer-causing agent). I think this should go back to the committee.
Steve Ela: The TR did note, and we received other comments, that there is no intent resin materials to go into the product; I think it is fairly clear about how these resins breakdown. There was a comment on that, but it was when the resins were being used incorrectly, and then yes, they will breakdown. But if these are used how they should be used, they do not breakdown. I think this argument can be made for many substances (ex. if we cut fruit up on a plastic cutting board, or the type of o’rings used in dairy). I agree with your thoughts, but I think it opens a rabbit hole of these food contact substances – how far do we go? Even though FDA defines this as a food contact substance, if we say it has to be on the National List, where do we stop? Do we require everything to be on the National List, it becomes really bulky. You mentioned styrene; that is certainly a big concern, but the way it is chemically put in and tied to things may make it behave much differently than for cancer causing.
Sue Baird: I am wondering, and I know the work you put into it and know your preference, if we could separate this into two different proposals. I think to the point that you made about resins – we need some real legal counseling on this before we can make an informed decision is valid – if we move to accept this proposal, what are we voting on?
Steve Ela: It is complicated. We have asked for legal clarification from the Program several times and have not gotten it, so we do not expect to get it. What I have tried to do in writing this is to say that you need a legal opinion, and depending on that legal opinion, that will determine how this should go. We are making a specification depending on the legal outcome. We could separate them. The question of the resins is the thorny one. If we separate them and come back, we are still going to have to deal with the resins. If this is voted down or send it back to subcommittee, I guess the next step is saying that resins have to be on the list, but that is a last-resort and not a real decision. I think we really need to kick this back to the Program at this point.
Kyla Smith: OFPA requires us to review ingredients and processing aids to put them on the National List. I do not think resins are either of these things. I do not think they meet the definition of a processing aid or an ingredient. I do think as a bigger topic here, it would be in the NOSB’s interest to have a determination whether food contact substances need to be on the National List. I think we need to have that bigger determination to keep us from grappling with the same issues going forward – whether that is coming from NOP or not. I think we should vote.
Carolyn Dimitri: As representative for consumers, there is something very unsavory in saying this is a definition that we should leave in the hands of the FDA and the Program. If we cannot say without question that these do not need to be on the National List, then how can we say that they should not be on the National List? I do not understand them, but it seems to me that resins should be on the National List.
Steve Ela: I respect your thoughts. Water, for example, is used – often softened by ion exchange filtration – we completely allow water without thinking twice about it. It is also used in pullulan, maybe citric acid, not like this is unheard of, and widely accepted by consumers, even in their own homes. I hear that you think it might be unsavory, but being a geologist, these ion exchanges are quite often in soils and that is quite normal. In my head, it seems to me that these resins are relatively inert. I would say completely inert, but I do not like to make those kinds of statements.
Wood Turner: I’m hung up on this. Are there other examples where the Board on the issue of food contact surfaces is making rulings on food contact surfaces versus the implication that if it is a food contact surface that it is out of our purview. We do talk about a lot of other food contact surfaces, but haven’t been on the Board long enough to be more versed on this.
Steve Ela: If it is a food contact surface, we have decided to not touch those as per OFPA. I’m going to say no, but I’m sure some stakeholders out there are shaking their heads and saying, “Don’t say that.” To me, it comes down to how much in the woods do we want to be?
Nate Powell-Palm: Wanted to highlight that there is a goal where we can move forward and keep progressing with the work. If I hear you right, I do not think that there is a lot that we can say differently about this if it goes back to the subcommittee or not. We have a lot of work to do, and we are going to lose some of our resources – you and Asa – and I think that we should vote.
Asa Bradman: There is some similarities here regarding concerns about BPA and canning products – situation where there is demonstrable leaching and is a valid concern. We have producers looking at getting phthalates out of their products. In some ways, I feel that materials that can leach are within our purview. This particular issue is very complex to me. I agree that our document outlines the issues and whether we are on position to deal with those legal issues. I appreciate the comments today from those representing consumers, and I think that is an important driver.
Kyla Smith: Was going to try to address Wood’s comment: Calcium hypochlorite (under chlorine) is a food contact substance. This brings me to my earlier point as to why I think it would be in our best interest…there are food contact substances that are processing aids that we would be required to review and put on the list. I do not think that resins fall into that category.
Brian Caldwell: I really appreciate this, and I’m sorry to prolong it. Steve, you outlined that the third option, which has not yet been a proposal, is that we require that the resin components be on the list, correct?
Steve Ela: That would be the next step on this.
Brian Caldwell: My thought is that it makes sense to me to do that. If these materials are pretty benign, they can be on the list and there is no issue. I think it could be done with sufficient lead time to not destroy the processed food industry. I would like that to be a possibility to be entertained, and my understanding is the only way that could be done is to send this back to subcommittee.
Steve Ela: As a note, I’m going to think that we would receive pretty much the same comments that we have received this time around. Secondary food additives were identified by FDA prior to food contact substances. There was a period of time when things were listed as secondary food additives because the listing of food contact substances weren’t a thing. FDA did not look back, and because of the way the regulatory process worked and timing, we do see some discrepancies in the way things are listed. Those listed as secondary food additives would have been listed as food contact substances if it had been an option at that time. I also have concerns that there would be huge push-back from the industry if we said this was going to be
Jenny Tucker: This process came to the Board because there was a conflict between certifiers. They had the same issues that you have, and they were struggling with decisions. It came to the Board because certifiers were struggling. Status quo right now is that certifiers are doing this – this is a source of inconsistency. We have not issued a noncompliance to any certifier on this. That tells you that we believe that the decision that the certifiers who allowed these things did not do anything illegal – they did not receive a notice of noncompliance. At this point you have certifiers doing it both ways, and we have not issued noncompliances. That leaves us in a position, like paper pots, where the Board will work on it and we will continue to allow the status quo while the Board was working on it. Or, if we do rulemaking, there would be a lengthy public comment process and likely an implementation period. Either continued allowance that is already in practice, or do rule making and provide a long implementation period. Really important to remember that certifiers are making these decisions every day.
Steve Ela: With paper pots, the Program was going to disallow use of them because they did not meet the newspaper definition, and you did not have authority to allow continued use of them once you determined that they were not technically allowed. The only way we were able to get around that is to note that if the plant was taken out of the paper pot, then the paper pot would be allowed as a recycled material. I do not see how you within your authority allow them to use in this case?
Jenny Tucker: Right now, they are allowed, and we have not issued any notices of noncompliances. This came to the NOSB after an interpretation was made by the program that the decision the certifies made was valid. Therefore, it is allowed until rulemaking would make it prohibited, if it goes that way. I think that certifiers made reasonable decisions. This is a hard topic.
Sue Baird: Steve, I heard your comment that there may be contact and sometimes not contact; is this somewhat analogous to boiler additives?
Steve Ela: Boiler additives where there is no interactive, then no they not need to be listed. They are defined as a secondary food additive. If would be using a boiler additive that does interact, then it would need to be listed on the National List.
Sue Baird: Is there a way to list this like that?
Steve Ela: The resins always have contact with the food. The question is if the resins have a technical effect on the food, or are they just there to hold the ions? My sense is that they do not have a technical effect.
Mindee Jeffery: I’m a little both/and on do we go back to subcommittee or vote on this? In the sense of representing what a consumer would say to me on this product, when I’m able to land it for the consumer that the FDA has authority over this and that is how we move forward on this, and I think that really works for consumers. I believe that if we vote this through, we are going to receive more information about the resins and then move forward from there.
Steve Ela: My personal response as a Board member is that I haven’t heard anybody in the certification community grouse about the recharge materials needing to be on the list. I think certifiers are in agreement on that. It really is the resins issue. There is no doubt to me that some of these resins are listed on the food contact list – that is clear – then it becomes legally again how the Program takes that and runs with it. I would have written this proposal differently if I didn’t come down on the side that it really is beyond our capability. It really is a legal issue. I think the certifier discrepancy is part of that. If we send it back to subcommittee, I think we are going to end up in the same debate and same scale of justice that we are now and we are still stuck. I do not see a way to create a unanimous on the Board either way.
Mindee Jeffery: I think it is a process. I agree that we agree on the recharge. If we go back to subcommittee, does it help us address concerns of the stakeholders that this Board stays really in touch with what is happening. We are okay with status quo right now. I think it is important for us to get this right. If we go back to subcommittee, can we get clarity from the FDA and regulatory format; can we get clarity?
Steve Ela: My short answer would be no. The TR said that essentially there is no literature saying these break down an adulterate food. Some of our stakeholders have said they do. Others have said they don’t. I think we could send it back without a quick fix.
Mindee Jeffery: My question is more on regulatory clarity.
Steve Ela: FDA has made their position clear. I do not think there is any more clarity on those issues by waiting.
Kyla Smith: The status quo is fine, but ultimately it’s not a great place for us to live in. We need a direction one way or another so that certifiers can be aligned. There seems to be some appetite to take it back to subcommittee if we can get some help. Jenny, are you able to speak to that?
Jenny Tucker: When we sent our original notice to the Board, we did share a specific interpretation. We got responses back to the certifiers that said that they did disagree with our interpretation, and it seemed they had a legally defensible basis. It would be helpful to know if you do send it to the Program, are you asking us to get back to you and you’ll make a decision? Or are you telling us that you’ve done what you could and we should just address it? Help us in your cover sheet. Making it more constrained than it currently is would take rulemaking. I do not see this as something that is a high priority right now given what we are currently working on.
Steve Ela: Could motion to send back to subcommittee, vote on the motion as it, or defer the vote to tomorrow.
Feedback from the NOSB is that the vote will be deferred to tomorrow.
2:30 PM ET: Handling Subcommittee (HS), Sunset Review
Agar agar

Kim Huseman: Public comments indicate overwhelmingly that in some form, agar agar is supported for relisting. Agar-agar is derived from red algae, the main species harvested being Gelidium (natural) and Gracillaria (synthetic due to processing), the second of which can be cultivated.
Some commenters said there is not enough commercially available non-synthetic or organic form; others said there is enough. One certifier noted 5 operations using organic agar agar. Used in dairy free shredded cheeses, hair care products, etc.
In the broader discussion of marine materials as it relates to agar agar, what is the Board’s stance? Work is needed on how it is listed in annotations.
No comments or questions from the Board.
Animal enzymes
Kim Huseman: Across the board there is support for animal enzymes and relisting. There are also comments that discuss the availability of organic rennet – products that come from organic livestock – and feasibility of using only product that has been derived from organic animals. Clear understanding that this may not be commercially available today, but challenge what are the barriers. There is a known entity that does have organic animals utilized in their rennet processing, but very small scale. These are considerations since last sunset: have they become organically available? Answer is no, but can we challenge this?
Ancillary substances: noted by one of the commenters did not investigate other ancillary substances; limited to rennet and egg white lysomes.
Finally, is there an environmental impact. TR says no. Commenter said that solvents and extracts are used in the production.
It is used for cheese without a good substitute – but could we challenge that with organic livestock production being required or have the research to understand that a bit better?
Sue Baird: The challenge with organic is that there are not sufficient enough processors for the organic version. Not that there are not enough organic animals.
2:45 PM ET: Lunch Break
3:10 PM ET: Handling Subcommittee (HS), Sunset Review
Calcium sulfate

Wood Turner: Calcium sulfate can be obtained from natural sources or synthetic sources. The listing restricts calcium sulfate to mined sources, and mined gypsum is the primary source. After crude gypsum is mined in open- cast quarrying or via deep mining, it is ground and separated. It is normally sold in pure form but may contain impurities of calcium carbonate and natural occurring silica. It can form as a by-product from many different kinds of processes, including from emissions from fossil fuel power stations. The material is Generally Recognized As Safe (GRAS) by the FDA.
Environmental Issues: Mining of calcium sulfate (as gypsum or alabaster) has exposed several public land areas, including Grand Staircase-Escalante National Monument in Utah, to extractive impacts. It is unclear the full extent of these activities to date, or landscape and critical area damage that could occur in the future. This question could potentially be addressed more fully in more current Technical Report (TR), as the most recent report on calcium sulfate is a 2001 Technical Advisory Panel (TAP), especially given that the sunset under consideration is the mined version.
A lot of feedback in past from the community – similar to what we are hearing today. Would like to see this retained, other materials available for use, asking for an annotation. In 2016, advanced for relisting.
Current feedback, certifiers and stakeholders suggesting there are limited number of folks using this product. Some of have reported that there are zero, but may be a geographic distinction. Some farm organizations have supported relisting. Other concerns that we need a TR, and that we should annotate this listing for coagulation in bean curd, specifically.
No comments of questions at this time.
Carrageenan

Jerry D’Amore: Written commenter pointed out that carrageenan is an extracted form of Irish moss and can be extracted from other red seaweeds as well. Two species are noted to have impacts on neighboring populations in the sea.
IFOAM allows carrageenan as a food additive without annotation.
We have discussed human health concerns more than anything else. The original studies were confused by evaluation of poligeenan, the degraded form of carrageenan. I take comfort in the 2004 FAO report that it is unlikely to be absorbed in-gut (after concerns about it in infant formula arose).
70% of commenters supported relisting. Nay-sayers had compelling arguments, and were strongly against it.
Summary of questions/commenter answers from public comment:
- Should there be an effort to outline best management practices for seaweed farming and harvesting? I do not know how we could control it, so how much weight should we give to that at all?
- Do seaweed farming practices for carrageenan production conflict with the proposed Marine Materials guidelines passed by the NOSB last year? It could, but not yet. The document seems like a thought-piece for future guidelines.
- Is carrageenan essential for production of organic products? Which products? It is not necessary for the production of any organic products, but highly desirable as it improve mouth feel in dairy replacement products.
- Are carrageen alternatives available to replace all current uses? Guar gum and xanthum gum seem to be interchangeable but there are subtleties in use.
- Would lack of carrageenan availability limit opportunities to produce vegan products? No, but goes to increasing their “appeal” due to emulsification/thickening.
- Is there new information on the safety of carrageenan? Original answer: health impacts are increasingly likely due to confusion between carrageenan and poligeenan. I have been counseled by several folks that I respect that I have to look at this more carefully and take into consideration the source of that information.
Discussion from the Board:
Asa Bradman: One thought that I had about this material, the idea of stare decisis [the legal principle of determining points in litigation according to precedent] that the use to grill supreme court nominees about precedent. A prior Board recommended removal of this. What level does it have to get to for us to potentially go against that decision. With sunset, we are reviewing them every 5 years. This is unique in that there was a recommendation to remove it. I feel like we have to respect that decision. When we review a new sunset, there can be new information available. This was a fairly concrete recommendation that did not get followed up on.
Jerry D’Amore: Would it be fair to say that the decision that you are talking about deals with the human health concerns?
Asa Bradman: Human health was driving factor [in previous decision].
Jerry D’Amore: When I made reference in my comments, it was about the human health effects, and we will discuss that.
Amy Bruch: One common comment: maybe we need to look at this as a synthetic substance, even though that’s outside sunset process.
Jerry D’Amore: In my reading, I sort of blew past that, but respect your thoughts on that. It appeared to be fairly well considered on the last go-round, but I will put synthetic on that list, too, for consideration.
Steve Ela: That was just preceding when I got on the Board. I think it was human health and essentiality as to whether there were alternatives available for it in addition to the human health. We cannot consider cost, but the program has to consider cost in rulemaking, and I think that the cost impact may have derailed it.
Jerry D’Amore: I would concur with that last statement entirely. As far as essentiality goes there are other things that could replace it, but functionality is hard to replicate. The human health concerns is back on the radar.
Steve Ela: A number of manufacturers have removed carrageenan from their products since our last recommendation. Are we down to the products that it is “essential” to now?
Jerry D’Amore: I have specific comments on that. The quote was, “Hey, it takes a long time to overcome bad press” and that it was the bad press at the time that cause producers to remove it. Also, a note that you have to believe in functionality. Nobody would eat this stuff because it’s delicious.
Mindee Jeffery: Mouth feel and how a product stabilizes impacts consumer perceptions. Do we keep carrageenan because we don’t want to keep shaking our soy milk?
Raising the question from pure mouth-feel to “what are our responsibilities?” And I agree with that. Previous Board voting to de-list has an impact on me.
Kyla Smith: Are we down to just continue; a lot of products don’t include [carrageenan]. Vegan marshmallows—do they need it? Do we need vegan marshmallows need to be organic? Many people would prefer to have organic products, but what organic products actually require this?
Jerry D’Amore: You closely mirror my call-ins on this one – you are on target with the marshmallow soup.
Mindee Jeffery: The public commenters that said something about if it’s in ice cream it might not be in the label—point I’m interested in. I’ve never seen an organic vegan marshmallow, I think it’s the gelatin!
JerryD’Amore: Specifically asking about the identification of carrageenan on the label?
Mindee Jeffery: Yes, I find some validity in the health concern folks. I am gluten free; like the market development. If there is a transparency issue I want to substantiate that.
JerryD’Amore: 75% were in favor of; 30% opposed were adamant, strongly not in favor and human health concerns and concerns about identifying it on the label are valid.
Glucono delta-lactone

Wood Turner: Glucono delta-lactone (GDL) is primarily used in the production of tofu, particularly in the production of silken tofu, and is generally thought to be the only material that can produce the physical and sensory components favored in that product. In tofu production, GDL serves as a coagulant. GDL can also be used as a curing or pickling agent, leavening agent, pH control agent and sequestrant. It is also used in feta cheese in place of lactic acid bacteria to reduce pH. Less tangy than citric acid, GDL slowly undergoes hydrolysis in water and converts to gluconic acid to produce a tangy flavor in food applications. GDL is Generally Recognized As Safe (GRAS) by the FDA.
Manufacture: There are a variety of ways GDL can be produced. The most common method to produce gluconic acid is called the Blom process, where gluconic acid is produced by fermentation of glucose syrups by Aspergillus niger. Sodium hydroxide or calcium carbonate is added to the fermentation process to produce gluconate salt. The gluconate salt is then isolated via evaporation, crystallization and then conversion to acid via ion-exchange. This process produces GDL via acid base reactions and fermentation (2016 TR, pg. 10-11). Other processes to make GDL involve oxidation of D-glucose with bromine water (which is not allowed by the National List annotation) and purified enzymes (TR 281-282).
GDL is >99% pure and has no ancillary substances present. GDL is often sold in formulation with other additives specifically designed for the application. These substances should be reviewed separately as they are not ancillary substances.
A little difficult to document environmental or human health issues. Some consideration that it might cause minor discomfort or back pain. No risk under environmental concerns. Original petition was for the coagulation of tofu, but not classified or annotated as such. There are several other coagulants that have been indicated as having similar uses – essentiality is unclear. During the previous cycle, the HS was thought to understand whether there were other uses of the material – feta cheese. From the community as a whole, there is strong feedback that in order to continue to include GDL on the list, we need to make sure that there is no GMO production.
Have not gotten as many direct responses to our questions as we would have expected.
How widespread use? Tepid response.
Excluded Methods – Nothing definitive in this cycle of comments. Hope to hear from the community in the fall.
Amy Bruch: I found one comment in particular that mentioned that the annotation was a bit limiting when it said it was prohibited from being produced with bromine water. Perhaps the annotation needs to exclude other production methods, as well.
Kyla Smith: Want to explain about why some certifiers may not have reviewed these – PCO does not certify as many handlers as other certifiers do – we may not have any producers of silken tofu. That may explain the difference of why some certifier report on these and some do not.
Tartaric acid

Steve Ela: Comments that it is essential. Important to adjust pH without use of sulfur dioxide. Alternatives are not sufficient. If material were prohibited, it would hinder people, as they spent years perfecting a synthetic-free wine. Main question was is there an organic alternative. Is there enough supply of organic wine to make this product? One person said there is not enough market demand for it, so it doesn’t exist. A lot of people said this is the chicken and egg thing – until you de-list, there won’t be enough supply to meet the demand. When is the time to de-list something to encourage the organic marketing. One person said there is absolutely no reason it could not be developed organically, but it would take some time for manufacturers to get up to scale.
Kim Huseman: I saw in one of the comments that this material is used to adjust the pH, but at times they do not have to adjust the pH – so, how often does it need to be used?
Steve Ela: I had not picked up until I read this – one commenter said they use it to adjust pH, and another said they do not need it to adjust pH. That is confusing.
Jerry D’Amore: The brics of the incoming grapes might have a lot to do with that.
Cellulose

Carolyn Dimitri: Certifiers and end users indicated that it is not widely used, but essential when it is used. Ancillary substances: One group reports that glycerin or glycerol is used, but not on our list. All comments indicated that cellulose sources other than wood pulp just do not work as well. No one answered #4. Everyone except for one or two people said this should be relisted. One group said that manufacturer should be done in a way that minimizes environmental impact.
Chlorine materials


Asa Bradman discusses chlorine materials in general: This is in some ways a complex topic. Chlorine materials overlap with many of the materials we are talking about when it comes to disinfection and sanitizing materials. Disinfectant does not require sanitizing – we should be careful to distinguish between uses. Chlorine materials are used to control pathogens. In many ways, we are going to say they are essential. Demand for them. One of the most effective tools to deal with FSMA. One of the most hazardous in terms of the environment. Need to do deep thinking about this, as they are much more toxic than many of the things that we might allow for.
Remarkable level of comments in this round. Thoughtful comments from BP, OWPC, CROPP, OMRI, etc.; there are some great reads in the current public comments on sanitizers in general. I know we are supposed to be talking just about the sunset here, but this is lodged in a much larger issue.
Chlorine compounds are hazardous to the environment and human health. Many ag workers have complained bitterly about bleach and other exposures. There can be severe respiratory and ocular effects. When we talk about this risk with contact with food, we are often talking about levels at safe drinking water standards. These materials are used in many different ways; for example, boot dips at 100 ppm and you can smell bleach in the room. The odor level is below the adverse health effects level, but may exceed an occupational health issue. I have tried to review occupational health of these materials, and there is very little out there. I’ve had enough experience with these that I know that even the label protections do not protect individuals – based on ventilation, exposure levels, etc.
There is one comment that we should be listing higher concentrations if they are being used in other settings than just the safe drinking levels in water. I think it would be good to include some of those other standards that are available. There are a lot of interest in what the alternatives are. We have talked about on the Board that there are a number of materials that have been evaluated by EPA and are listed on the SCIL. In talking with folks in the field on this, it is kind of a wild west environment in some ways. It is not clear how different sanitizers and disinfectants meet the goals of food safety. When there are materials that address different food safety issues, there is a tendency to rely on those. If we were to suggest citric acid or some other approach, if there was a food safety outbreak, that could make any food manager hesitant to make that choice. There is a status quo and use of materials that we know are safe.
When we look at the relative efficacy for unique pathogens, particularly around biofilms, would be interesting.
We asked the panel if there could be an approach to healthy microbial populations that are not pathogens and therefore discourage pathogenic bacteria – I have gotten the answer of no. In soil, there are ways, but in a food processing place, given the colonization and re-colonization and the need to clean and then follow up with disinfecting, there is really no way to out-compete a pathogen.
In many ways, I feel like we are stuck with the chlorine materials. Even people I have talked to who are responsible for HAACP plans (we are probably going to relist these). I would suggest that we all read through the comments that have been submitted. I think there was great thinking that have gone around.
I also wanted to highlight the OWPC comments on the framework around sanitizers. Reminder, beyond the chlorine compounds, the sanitizer issue is front and center in many ways.
Potassium hydroxide

Steve Ela: Wide-open listing except for prohibition for peeling (exception: peaches). Stakeholders prefer a listing for what is can be used for versus what it can’t be used for. It is highly corrosive; severe burns are possible. There are enviro concerns in case there is a spill or disposal; risk to bodies of water. It is a hazardous material and is toxic. Is it essential? Are the various uses essential?
Other commenters said it was essential as anti-foamer, pH adjuster; other products are not sufficient because it is more soluble than others. If prohibited we would need to identify something with the same functions.
Potassium lactate

Jerry D’Amore: Used as an antimicrobial agent and pH regulator. Confirmed as GRAS. FDA does not authorize its use in infant foods and formulas. Does not appear to cause human health concerns. Does not seem to pose any real environmental concerns. EPA adds it has low potential to existing the environment. Not accepted by Canada, IFOAM, JAS. It has been allowed for use in organic handling since 2004.
Between both oral and written comments – less than 10 responders – most in favor of relisting. Does not appear as a heavily used material.
Asked what distinguishes from Sodium lactate – potassium lactate offers similar antimicrobial function as sodium lactate but without added salt or salty taste. Attractive to consumers looking for less salt intake.
No questions or comments at this time.
Silicon dioxide
![]()
Kyla Smith: Used as anti-caking agent in several foods; adsorbent, carrier and de-foaming agent. Most of the comments were in support of re-listing. Some comments asked Board to look into original annotation that was passed because there was a difference in recommendation and actual annotation that was passed by NOP.
Some comments said there are limited applications where substitutes exist; but not all applications. Comments also spoke to % and ratios that needed to be used; it’s not 1:1, which creates problems in needing to use more organic rice hulls (affecting taste, etc.).
Reliable commercial availability – None.
How prevalent is its use as a de-foamer? One certifier, 1-5 of the materials mentioned its use as a de-foamer. Some use as de-foamer.
Its use for other allowed purposes had more comments: supplements, beverage mix powders, beer, used commonly as an ancillary anti-caking ingredient, in nutrients and minerals, etc. Very common as anti-caking and flow agent (particularly in spice industry).
Sodium lactate

Jerry D’Amore: Used as an antimicrobial agent and pH regulator. Confirmed as GRAS. FDA does not authorize its use in infant foods and formulas. Does not appear to cause human health concerns. Does not seem to pose any real environmental concerns. EPA adds it has low potential to existing the environment. Not accepted by Canada, IFOAM, JAS. It has been allowed for use in organic handling since 2004.
Between both oral and written comments (less than 10 respondents): most in favor of relisting. Does not appear as a heavily used material.
Why has JAS, IFOAM, and Canadian standards prohibited the use? Fairy uniform response – synthetic material and there are Non-synthetic and naturally derived alternatives.
No questions or comments at this time.
4:25 PM ET: Handling Subcommittee (HS), Petitions / Discussion Documents
Zein, petitioned for use at §205.606

Jerry D’Amore: The NOSB was petitioned in February of 2020 to consider zein, otherwise known as “maize protein”, “protein coating” or “confectioner’s glaze”, for addition to the National List. The full petition may be found here. The petitioner, Flo Chemical Corporation, asked for inclusion of zein under “Nonorganic agricultural substance[s] allowed in or on processed products labeled as “organic” (§ 205.606).” The NOSB asked for a technical report which was produced in January 2021 and deemed sufficient in February 2021.
Response was light – not one single comment in favor of the position. One responder did not indicate yay or nay. The was one strongly worded comment against the use of preservatives for shelf life.
- If zein is made from cornmeal that is wet-milled, how much (if any) sulfur residue is left in the final product? Wet milling steeps the corn in a hot water solution for 24-48 hours. The solution has been held at somewhere between 0.1-0.5% sulfur dioxide. None of the concerns had any precise numbers to share – thought none if any. Given the specifications and labeling requirements, presumably any product left would not have a functional effect.
- What are the hurdles to achieving organic zein? Sufficient quantities of organic corn meal. Would we be stifling innovation if we allowed this petition? I am not sure that the demand for the product – organic Zein – is strong enough at this point to push production.
- What sectors of the organic food market would benefit the most significantly from the addition of zein to the National List and how much will shelf-life be improved? Question is a bit leading and provoked and well-thought out and lengthy document regarding the need for anything that prolonged shelf life. The petition has stronger points – hydrophobic and best for pharmaceutical pills and is a barrier. The most comment response was, “I don’t know anyone who wants it.” Another response, “We do not know until we try.”
- Do we need to revisit the classification as a non-synthetic, or is the established precedence sufficient rationale? Provoked strong comments both ways. The push and pull of what we are trying to do – there is no commercially available organic zein today – what does that mean to us in considering this petition? There is no market for it. If we allow it to go forward, will there be a market for it, and will that be good for the people we represent?
No questions or comments from the Board at this time.
Fish Oil Annotation, re-issued Discussion Document


Asa Bradman: Another complicated marine materials issue; came up w/ sunset review of fish oil last Fall. The HS thought that to renew sunset there needed to be an examination of environmental impacts re: harvesting fish and using them for organic production. The sunset did pass; there are some objections to the actual listing on the NL. How do we protect the ecology of fish through this? If we eliminated this substance it would make the discussion moot—but it was re-listed.
Last Fall Tom prepared a suggested annotation to modify what is acceptable for this material. Came up w review of fish oil annotation based on definitions from FAO and NOAA. Oil must only be extracted from fish that were being harvested sustainably and not endangered.
Comments noted that this was inconsistent for certifiers because of different standards for NOAA and FAO. Other comments that there are some populations that are not covered by these bodies but are over-exploited. DSM and GOAD. Are the industry groups that commented.
Scientists from NOAA office and Monterey Bay scientists, MSC folks commented around integrity of use. Those discussion formed this discussion document. Came up with 3 alternatives which were reviewed by NOAA scientists.
The options:
- Option 1 Current Proposal: 205.606 (e) Fish oil (Fatty acid CAS #’s: 10417-94-4, and 25167-62-8) – stabilized with organic ingredients or only with ingredients on the National List, §§205.605 and 205.606. Sourced from fishing industry by- product only and certified as sustainable by a third-party certifier.
- Option 2 Current Proposal: 205.606 (e) Fish oil (Fatty acid CAS #’s: 10417-94-4, and 25167-62-8) – stabilized with organic ingredients or only with ingredients on the National List, §§205.605 and 205.606. Sourced from fishing industry by-product only and certified as sustainable against a third-party certification that is International Social and Environmental Accreditation and Labeling (ISEAL) Code Compliant or Global Seafood Sustainability Initiative (GSSI) recognized with full utilization of said scheme.
- Option 3 Current Proposal: 205.606 (e) Fish oil (Fatty acid CAS #’s: 10417-94-4, and 25167-62-8) – stabilized with organic ingredients or only with ingredients on the National List, §§205.605 and 205.606. Sourced from fishing industry by- product only and has either a green or yellow Seafood Watch rating or is eco-certified to a standard recommended by Seafood Watch (https://www.seafoodwatch.org/).
We have gotten a number of comments on that, and they were varied. In the proposal, we are talking about
Fish oil as a secondary from harvested fish; not fishing solely for oil. By catch is deriving it from unintended species that were captured and killer versus target species. Speaking to sustainable harvest we are only talking about secondary harvest, not bycatch.
There were concerns for all of them but strongest support for #1 & 2. People preferred #1, but people commented it was too vague and left room for issues. By relying on 3rd party certification we are relying on a standard that is outside the USDA’s control, and the USA’s control. Issue of greenwashing. Possibility of excluding smaller scale producers who cannot afford 3rd party certification. These issues remain with any option, but are of least concern with option #2. Sea Food Watch standard would allow the use of “green” and “yellow” but using yellow fish is a concern.
PCC thought they were all inadequate, but certifiers and industry folks ( DSM/GOED) leaned toward # 1 OR 2. Other comments felt that #2 is work-able; Friend Of The Sea felt that there was a concern that they would be excluded bc they represented smaller groups and didn’t have “big league” sustainability certification.
Final comment: need to have a certified aquaculture standard. If we did, we could source material from “organic” fish. That would make this potentially moot as we would have an organic source (CROPP mentioned this). Those are the major points.
Discussion?
Kyla Smith: These options are focused on certification; is there an over-arching accreditation scheme that would wrap them into a particular ISO standard (w/ 3rd party certs)? Are there specific cert schemes called out a problem; what if they change their certification down the line and it’s tied into the annotation? Being vague leads to some inconsistencies, but being specific is also a challenge.
Asa Bradman: Part of the idea behind #2 option. Those are some broader accreditation certification bodies where the others are vague. But yes, they could change. Also, some components are voluntary and others are mandatory; we’d probably want to limit things to the mandatory. But problematic to limit standard to outside body. But we don’t have the resources to deal with it, at least until we have an aquaculture standard. I don’t believe there is an ISO standard for this.
Carolyn Dimitri: So fascinating. Whole fishing scene is fraught with so many problems everywhere you look. These comments build on a little bit what Kyla was saying: third party certifier can be a little too vague. It leaves room for anyone to open shop and become a third-party certifier without having any bite. Offer a list of legitimate third-party certifiers – should be some kind of minimum vetting process, so you could feel like something is being certified other than just a stamp. .
Asa Bradman: The Seafood Watch one might be the best if we took out the yellow category, although concerns it that it is desk-based. Others do actual inspections. The other thing is that they have a public-facing consumer face for many fish, but they also address many fish. That might be more accessible – US based – do not think they have the global reach that these other organizations do.
Carolyn Dimitry: Essentially, these 2 organizations would be in charge of vetting certification systems, and we would trust them?
Asa Bradman: They wouldn’t vet them for us. They would accept an attestation that they were getting stock from an organization that meets these standards. It would be to some extent honesty based, but it would still be a step.
Wood Turner: One of the beauties of this process is that we put these concepts out for consideration and get feedback on them. It helps to clarify and get feedback. Funny that you raised the PCC feedback – they seemed to be defaulting to Option 3 – when I was feeling that Option 2 seemed much stronger. Seafood Watch is great, but to your points, it has its own issues. I end up landing on this – what this is telling me is that we do need aquaculture standards in organic – period – the end. That is what is needed here. In a lot of different areas, this is a reflection of the continued need to define these things more clearly and stand by them. What is beautiful about organic is that we have the force of law behind them. We all know that across the board certifications are fundamentally different because of that point. I know this is a big can of worms, but wanted to put it out there.
Sue Baird: Just to the point that you said it would be somewhat a point of trust with third-party certification, there is somewhat precedent on that. We accept OMRI to review our inputs that are used in organic production. We accept CDFA when they verify that there are no pesticides applied for three years.
Steve Ela: I have to think on that a bit. We have run into this a bit with the Safer Choice List – that is another agency that makes decisions without any public comment that we then take up and use. When we tie our fate to some of these other standards, we give away our stakeholders’ ability to comment on it. Recognize that we cannot do everything. That is where Wood’s comment comes in – where aquaculture standards would come into play.
Asa Bradman: Tom originally thought that we should use FOA and NOA – one for domestic and one for international products – but it wouldn’t be uniform. This discussion is around trying to make it uniform. One idea is that if we do come up with a proposal, we could have it expire once there are aquaculture standards.
No additional comments or questions from the Board at this time.
5:00 PM ET: Adjourn
Wednesday, April 28, 2021: NOSB Meeting
12 PM ET: Call to Order, Agenda Overview, Introductions
Jennifer Tucker, National Organic Program Deputy Administrator, gives some opening remarks and calls the meeting to order.
Mae Wu, Deputy Under Secretary of Marketing and Regulatory Programs, gives some introductory remarks concerning the activities of AMS. This administration is finishing Origin of Livestock (OOL) and Strengthening Organic Enforcement (SOE) and tackling the problematic Inert Ingredients. The pandemic response includes getting assistance out (COVID-19 stimulus package); grant programs. Organic production is useful for climate change. Excited about WH nomination of USDA Marketing and Regulatory Programs undersecretary, Jennifer Moffitt.
Roll call is performed. All current NOSB members are present.
Organic Producer Seats:
Steve Ela (January 2017 – January 2022)
Nathan Powell-Palm (January 2020 – January 2025)
Amy Bruch (January 2021 – January 2026)
Logan Petrey (January 2021 – January 2026)
Environmentalists / Resource Conservationists Seats:
Asa Bradman (January 2017 – January 2022)
James R. “Rick” Greenwood (May 2018 – January 2023)
Wood Turner (January 2020 – January 2025)
Consumer / Public Interest Advocates Seats:
Sue Baird (January 2017 – January 2022)
Carolyn Dimitri (January 2021 – January 2026)
Brian Caldwell (January 2021 – January 2026)
Handlers / Processor Seats:
Kimberly Huseman (January 2020 – January 2025)
Gerard D’Amore (January 2020 – January 2025)
Retailer Seat:
Mindee Jeffery (January 2020 – January 2025)
Scientist (Toxicology, Ecology, or Biochemistry) Seat:
NOTE: the current Scientist seat is vacant, meaning there are only 14 NOSB members at this time.
USDA Accredited Certifying Agent Seat:
Kyla Smith (January 2021 – January 2026)
NOSB Secretary’s Report
Mindee Jeffery, NOSB Secretary accepts the minutes from the Minutes from the Fall 2020 meeting.
NOSB Chair’s Report
Steve Ela, NOSB Chair, reports on the activities of the NOSB.
Encourage folks to apply to serve on the NOSB. There will be several seats becoming open. Want to really encourage diversity – the Board is fairly representative of the organic community as a whole right now, but we always talk about the need to diversify the community and make it open to all growers –(particularly racial and geographic and gender diversity). Please apply. If you have any questions, reach out to Michelle Arsenault or [Steve Ela]. There are a number of other organizations that can help you, as well – NOC, OTA, others. We are interested in getting the best people. If you have applied before, please apply again – the fact that you weren’t chosen last time does not mean that you won’t be chosen this time.
This is our 3rd virtual meeting. It has worked well in some ways – allowed people to not have to travel, spend money, webinars for public comment have allowed more people to comment – but the in-person meetings do provide a richness and allow us to meet and know each other as friends. Keeping our fingers crossed that the fall meeting will be in Sacramento. Working on keeping a livestream portion of the meeting. Our goal continues to be transparency and access to all stakeholders. Hoping this can address some of the issues with timing of the meetings.
Nominations – Next Tuesday, we are having Coffee with the Board Webinar – talk with Board members, ask questions – informal – anyone who is interested in the Board and how it works, please join us.
We always get comments from stakeholders regarding work agenda items and tension between Board selecting our own WA items and the NOP only allowing us to work on certain. Great arguments on both sides – time commitment – not having the Board work on things that aren’t actionable, but also allowing the Board some free will. Not sure how to solve it, but perhaps it is time to explore a bit of a mix. NOP with oversight, but allowing the Board to select some things that they’d like to work on. Want to make the time on the Board management and not increase it. Hard to look at the big picture items even with the current work agenda items, but we hear from stakeholders that the job of the NOSB is to look at those big picture items.
Excited to hear about the issues of climate change coming down from this administration. We know this affects all of us. Critical for agriculture to really look at this. Organic agriculture is so well suited to dealing with this issue – both through sustainability, reliability, ability to persevere through difficult events, and also, we are a volunteer group – we signed into it – we have already opted into supporting a way of growing food that really addresses those climate change issues. Hearing this language and seeing this as a focus makes me really excited. In some ways, I am jealous of the Board members who will get to work on these issues.
My fifth year, I came onto the Board not really going to NOSB meetings, not completely familiar with the process – it is such a neat process, and I respect the wisdom of our stakeholders in giving us input. I have talked with many stakeholders who have participated in giving input to the Board, and with new members, I want to stress how important that is. We have a 5-year snapshot of this. I want to shout-out to our stakeholder community about how valuable you are in reminding and educating the Board on past precedents and processes. You are so important. Thank you to all our stakeholders.
Looking forward to this meeting. We do not have a lot of votes, but discussion will help guide us in the fall. Public comments were excellent.
National Organic Program Report
Dr. Jennifer Tucker gives 35-40-minute update on the Program’s current work and projects. The presentation can be found in the Organic Integrity Learning Center (OILC) and the slides can be found reproduced here.
Dairy compliance has expanded into broader livestock enforcement work. Some of that we do not celebrate our successes publicly, but they are happening.
Tucker discusses the ongoing import fraud work. We ended our US-India recognition agreement – had been in place since 2006 – we had authorized the India government to do that accreditation work. Found that India’s internal control system was not adequate to meet the needs of that recognition agreement. January sent a letter laying out a transition plan to end that agreement. By this July, anyone recognized under that agreement must apply with a USDA certifier. We are seeing impacts in the market. This was done to protect the market. Part of our import oversight work.
We have also been seeing some continued deepening with our relationship with U.S. Customs and Border Protection (CBP). We meet with that group regularly. Enabling a lot of activities that we did not have access to before. We have a staff member that works in CTAC, which allows us to, for example, send very specific directions to port officials. If there is an operation that has an open investigation, we can put in rules that if someone sees something coming in from the boarder from that entity, they can send it back to us. Something we have not had before.
Access to ports for certifiers and how to do targeted sampling and testing at ports is now in place.
There is a lot going on in enforcement. All of that continues to deepen the control systems within the NOP and with our certifiers. The larger NOP presentation goes into some more quantitative markers; so we can continue to do the follow-up work with certifiers.
Rulemaking is a priority. This is a goal of setting standards. They are important, and we do support continuous improvement. Wanted to give an update on the rules that are in progress, but also thought it would be instructive to talk about what makes rulemaking successful. We have some good case studies now. I think this could help all of us in how we frame regulatory needs for an administration. Learning what works in rulemaking makes us all better at continuous improvement.

The rules in progress:
- Strengthening Organic Enforcement (SOE): This rule is a key program priority – writing the final rule now – over 1500 public comments that came in last fall. Anticipate that rule entering the clearance process later this year – enters into many offices within USDA, including Office of Management and Budget. We have a team that has been working hard on drafting that final rule. Moving ahead nicely.
NOTE: For more, read Cornucopia’s 2018 white paper, The Turkish Infiltration of the U.S. Organic Grain Market (PDF), and the 2019 report by Anne Ross, JD and John Bobbe, Potential, Failures, and Pitfalls of the National Organic Program in Getting Control of Organic Grain Fraud.
- Origin of Livestock (OOL): This rulemaking is taking a long time but the rulemaking process is working. We had said publicly earlier this year that we did plan to move ahead with a 2nd notice for public comment to supplement the 2015 proposed rule. There is a notice that right now is at OMB. It will activate another public comment period. Being done to seek feedback on specific parts of the rule – provisions that were not part of the 2015 rule. OOL has taken a long time, and it is working. There were things in 2015 that people suggested that was great feedback, but because not everyone had a chance to comment on it, there is a new comment period being published. That is how it is supposed to work. This will show up in the Federal Register as a Notice. This is a high priority for the agency. The natural next step would be a final rule. I said that before and we got legal feedback that said we had to take another step. I’m not going to make the same mistake – we need to see what happens in public comment. This is a high priority and we are moving quickly.
NOTE: The delay in the OOL rulemaking contributes to the ongoing organic dairy crisis, with many family scale organic farms barely staying afloat or already out of business. Dairy consumers can help by using Cornucopia’s Organic Dairy Scorecard to support ethical family farmers. Interested readers who want to learn more about the origin of livestock issue can also check out Cornucopia’s comprehensive report: The Industrialization of Organic Dairy.
- National List Rules – One of the changes we’ve made over the past few years is to move along National List rules faster. I know the interest tends to be most with practice rules. National List rules are very important – part of implementing recommendations as a Board – does take time to get through the system. We do have a commitment to move forward with an advanced notice of proposed rulemaking on inerts. As Mae shared, she knows what inerts are and why this is so important.
Dr. Tucker than discusses what makes a rulemaking project most successful. Four factors we believe that make rulemaking projects successful:
- Align with the Act (The Organic Foods Production Act (OFPA). Comment that this is one of the reasons we have been able to sustain momentum with SOE. Great example where rulemaking ties closely with the regulation act.
- When rules address needs across the most community support. For example with the OOL rulemaking: Community really joined together in 2018 around this priority in a way that we hadn’t seen before. Planets aligned. Lots of communication on why it was important across the sector.
- Will resolve known market inconsistencies. Dr. Tucker outlines how the OOL is another good example of this factor: Reasons why you want a goal – consumer expectations & competitive fairness for producers. When I started hearing about OOL when I first joined the program, it was explained as a consumer expectation thing. I was at a NOC pre-meeting, and I was sitting next to a consumer advocacy lead. We were talking about OOL an she said to me, I get why it is important to them, but consumers do not really get this stuff. I thought that was very telling. Very detailed rule and expecting consumers to understand it or rally around it… When the community started selling OOL as a competitive practices issue, that is when it got sold. Think about if you have the data to support the fact that consumers care about this. That takes some pretty detailed questions in consumer surveys to show that. This is when from the inside I saw it click. My goal is to share with you the NOP process and how this plays out.
- When the economic benefits are clear. Dr. Tucker used the SOE rulemaking as an example: We tried something different in the economic analysis when we talked about deterred fraud. Anytime we have numbers helps. Cost benefit is part of the dynamic. We often hear that organic is voluntary and cost benefit shouldn’t apply. Right now, it does. All of the features of the Administrative Procedures Act really matter.
Those are examples of factors that contribute to success. Also helps explain when things aren’t quite as successful. For example, the Native Ecosystems recommendation is one area that [may not be as successful].
For Native Ecosystems there have been a number of public comments on the importance of pushing that along. Parts of that recommendation that aren’t covered by the OFPA. Automatically going to slow things down. If Congress did not authorize something that is being called for, particularly if it is going to have economic impact, that is going to raise a bunch of questions. Strong champions for it in the community but also some groups that have hesitation regarding the barriers that it will create for entry. Economic benefits – hard to articulate what the economic benefits of that rule would be. When you are thinking about what your projects are, trying to think about framing them in those terms, should be the goal.
Dr. Tucker notes the need to highlight how much has been done. More than 600 recommendations have come from the NOSB. NOP has implemented more than 85%. It’s not always rules – sometimes guidance, training, etc. There are a few that have not been moved forward, and we have forwarded a lot of those recommendations and acted on those.
What are some of the best ways to communicate our priorities to a new administration?
- Organization letters that reflect membership consensus.
- Sign-on letters that explain impact and provide concrete data.
- Letters that substantiate cross-sector support. (Looking to have groups from all stakeholders agreeing.)
- Be clear, honest about the “as is” and why “to be” is important.
- Think through who can act!
Examples: A few years ago, [the NOP] started to get some letters in on gene editing. The letters said that the NOSB has recommended that gene editing be prohibited, so you need to do that right away. I got a call asking if gene editing is allowed? We explained that it is already prohibited (if you read the regulations, you can see that). That took a bit of work to convince people that it is prohibited. It’s important to provide “as is” context on what the current state is when you are describing the current situation. This is where containers and hydroponics provides another example; it is important to openly communicate about the “as is” in a fact-based way. It is better to be open and honest about the whole picture up until this time and then talk about what you think the end should be. Selective story telling doesn’t help. Lay out all of the facts.
What happens after a communication is sent?
- Assigned through “Controlled Correspondence”
- Many hands across USDA review
- Gets priorities and different perspectives on radar
When you finally get a letter, it may say we are “evaluating priorities” or looking into things – it may not feel that you got the response you were looking for, but there is value in the process. By the time you get that response, a whole lot of people have read that letter and heard your request. That is why I think we had such a good response on OOL: a whole lot of letters came in and a whole lot of people read them. Process helps get the priorities and all of the different perspectives on the radar.
There are some things that are important and are going to be constrained by the pipeline. Talking about that USDA review process – we are competing for the attention of a lot of different people to get their attention and move things forward. The thru-put during the clearance process and thru-put at the office of OMB requires prioritization. Thinking through the impact – how to package and sell rules in order to be prioritized is part of the framework and process.
Example of four rules that comes up on the things that we haven’t addressed yet:
- Apiculture
- Pet Food
- Mushrooms
- Aquaculture
Knowing what we know about the rule pipeline now and how to “sell” rules, we might have considered thinking about all of those rules together. Might be different stakeholder groups, but all around market development – might have been interesting at the time in how to think about those as a package deal. Pet Food feeds into “really, organic pet food? They didn’t think we were elitist enough?” [Pet food] is really actually a livestock rule; it would help the LS sector of the organic community by making the maximum use of animals. I think if it had been framed that way, it might have been looked at differently.
A lot of interest in standard development, so I wanted to pause and talk about this. I know folks are frustrated about the NOSB recommendations that have not moved forward. Wanted to share some of the ways and steps that might have moved some of that forward if framed differently.
Dr. Tucker then goes on to discuss NOSB board nominations (the process through which new Board members are selected): Want to remind everyone of the all for nominations to the Board. If you do not succeed at first, please try again! Getting the right combination of the right people at the right time is really important. Presentation on May 4, 2021, Coffee with the Board. Please take advantage of this.
Dr. Tucker describes an upcoming funding opportunity related to the Human Capital work: This is a Program priority. We will be issuing, likely early next week, a call for proposals which will be distributed through Organic Insider [The Organic Insider is an email notification service that the AMS National Organic Program uses to send out program updates to the organic community.] There are multiple candidate projects that could be funded as the Board has been working on this project, we have been creating this funding opportunity. Getting feedback from certifiers, ACA, IOIA, as well as from industry and community groups. Will be a multiple award thing. We are going to be using a tool called a Cooperative Agreement – only enter into with a nonprofit – 501(c)(3) has to lead – to encourage teaming with a focus on equity and diversity. Encourage you to consider how you will broaden your diversity. How do we encourage groups to join who have not joined before. Appreciate all of the work that NOSB, ACA, IOIA have put into this project.
Questions from the NOSB members about the NOP presentation follow:
Wood Turner: We’ve heard several organic stakeholders voice their concerns that access to the organic movement and organic certification has not been equal across racial groups. Several NOSB members, including myself, have voiced our agreement with the need to advance diversity, equity, and inclusion in the organic movement. In addition, the current administration has stated that racial equity is a priority.
We have to do better – not because it is a priority of the current administration, but because it makes us better.
It would seem that we could easily begin to address this issue by ensuring that USDA NOP resources are available in languages other than English. Currently, on the NOP Documents and Resources Available in Spanish page, the link to the USDA Organic Regulations in Spanish links to an English version of the regulations, and the link to the NOP Handbook in Spanish is broken.
Can you tell us more about the Program’s plans to address this issue?
Tucker/NOP: Next week, we are going to be issuing an Organic Insider that highlights a bunch of Spanish resources and the organic regulations in Spanish, as well as the Handbook. We had invested work in this a couple of years ago, but we were not in a position to keep up with the content. We will get that information out and clean up those old links. This can help. We know that Spanish is important, and there may be other languages. This was an important step for us to complete.
Human Capital issues: The call for proposal is going to emphasize equity and diversity – we are going to have a concrete way of doing projects that support equity and diversity. Encourage teaming relationships.
Diversity of the Board is a challenge because diversity in the organic community is a challenge. The Human Capital work will not have an impact in the next 2 months, but may have an impact in the next 2 years. I would encourage everyone on the line to pick up the phone and call someone and encourage them to apply to the Board. I challenge all of you to do this. Who are some people that it might not occur to you – think of a broader poll.
Rick Greenwood: A couple of weeks ago at an executive meeting, I mentioned the form letters that you received. You talked about that a bit; wondering if you could go over that a bit with the audience.
Tucker/NOP: Form letters have value, both in public comment and for rulemaking. Form letters help communicate the # of people that may feel the same way. Anytime someone does original writing –a more personalized and tell the story of the farm or business – that makes a difference. Whenever there are form letters, we catalog how many people felt this way. Can be more effective to have a sign-on letter where many people sign on – has as much or more value than 200 of the same letter that has to be uploaded by hand. Anytime you can make things a bit easier for civil servants, it makes a difference. The least effective ones are ones where people on a website click a button and it automatically sends an email into my system. Help us help you.
Kim Huseman: Thank you for information around SOE, as well as the information the NOP put together in a separate webinar. Since that time, the information went into effect…we are in the middle of a pandemic. India’s COVID intensity has been a highlight. Suez Canal is an example of freight that is a legitimate concern. Is the NOP taking any kind of stance on feed being certified?
Tucker/NOP: How do we facilitate legitimate trade? The timeline that we laid out for India was purposeful. They need to apply for certification by July – not completed certification. That allows us to look at certifiers to ensure they are managing those applications appropriately. We think this is good model that allows us to get more visibility on what is happening in the market. We are going to keep an eye on the deadlines;– we are not ready to move any at this point. We will see what happens. India has made some changes in their oversight program, which we welcome, but that also has changed some of the supplies coming out of India.
I want to be very clear: our first priority has to be protecting the integrity of the seal and fair and competitive marketplace. We are starting to hear that some businesses are interested in applying for a temporary variance to not be required to feed organic feed due to supply issues. It is long-standing that temporary variances not be granted for feeding non-organic feed to organic livestock. This is in our handbook. That type of request has been received in the past and has been rejected each time. We believe the approach we are taking with India is the right one.
Amy Bruch: In addition to what Kim Huseman said, can you comment on what you anticipate seeing with the changes being made in India and many of the countries that are rising in their increase in export; where these countries have substantially different or no standards…
Tucker/NOP: We are much better able to see where the growth is as it is happening. There are some certifiers that have started using import certificates. That is going to help us and allow us to get ahead of the curve in seeing what the control systems are. Even if a country does not have standards, those businesses can be certified to NOP standards by a certifier. I have heard that it is hard to get a certifiers in certain parts of the world right now. If we do see some supply constraints in light of that, protecting the seal is our top priority.
Kyla Smith: Thank you for the candidness of where things are in the rulemaking process and encourage the Program to continue doing that so that all stakeholders understand and are on the same place.
Asa Bradman: Concerns about need to move ahead on List 3 & List 4 inerts; reflects an obligation to the community and to the Board – respectful of the Board process to ensure that we do not spend time on subjects that we shouldn’t. There was a lot of drama and hours spent on inerts that we shouldn’t have. Good to hear that is moving forward. Similarly, with OOL.
Native Ecosystems and the “needs” of OFPA: We need to do a cost-benefit analysis; there are ecosystem services that can be quantified. It also reflects values, and a lot of organic industry systems are based on values, not dollars. I think that is something as an agency that we need to consider.
Tucker/NOP: We need both the values and the things that are in the administrative procedures act seriously. Someone is going to ask me about containers…
I’m sensitive about the Board’s time – there are a lot of you that would like to work on containers – how do we do that in a way that will end up yielding a product that can be moved forward. I think we would really need to talk through if we have a commitment to move forward. That is going to take some buy-in – how do we get from a discussion on a Board through a rulemaking process – what is the Board willing work on, and what does that look like? I don’t want you working on things that I cannot do anything about, either. I do not want to be beat up years down the road about why we haven’t moved forward when we told you that we weren’t going to move forward.
Asa Bradman: I think that if we really want to diversify the Board, we have to make it accessible to people who do not have the resources and time to do so for free. I think that is really important.
Carolyn Dimitri: Competitive Practices concept for putting forth a new rule. Always challenging to talk about CP in cases where you do not have solid data. Having looked through many of the comments to the federal register, I can often see the one-sidedness. I have also read some of the federal notices from the NOP, that look like they could be strengthened. Is there any effort to work with NASS or other organizations to get additional data?
Tucker/NOP: I do think the organic data initiative is very important. SOE: more data is going to be required into the OID on acreage information – that should be a big help. The data is a challenge. I do think the NASS data is helpful. Letters from livestock producers in case of OOL talking about how much cows cost and what are the price differentials helps. We will always want more data.
Nate Powell-Palm: For the past 10 years, I’ve been an organic inspector – when I travel around, I will hear producers say that the standards in OR are different than those in PA – and I say, “No, that is the most wonderful thing, they are the same across the board.” I worry that the consistency is compromised by the backlog of recommendations. With them stagnating, we are left in the certification world without clear standards based on those recommendations. Do you believe that OFPA made the USDA the ultimate decision maker or the organic community. I would like to hear your ideas behind that.
Tucker/NOP: What a unique framework we have in organic of the public:private partnership. Anyone can go to see what the organic standards are. That is working within a framework of federal government to protect the process. There are a lot of gates to rulemaking, and as a citizen, I am glad there are. I’m glad the governing process is hard. The community owns the standards and the act owns the standards. Our job is to make that both/and. I think my answer is YES – let’s celebrate the diversity and different views on the process.
Steve Ela: Personally, as a grower, concerned about that lack of consistency in the three-year transition requirement.
This is an issue that cuts across many different production systems, including greenhouses, containers, sprouts, mushrooms, poultry and more. As you know, several stakeholder groups have commented repeatedly to the NOSB on this topic to express concern, including the National Organic Coalition and the Organic Farmers Association and the Accredited Certifiers Association. Stakeholders have expressed concerns about the lack of a level playing field for farmers, as well as challenges for certifiers and the issue of certifier shopping given that some certifiers are requiring a three-year transition when others are not.
I understand that the ACA has devoted substantial time and effort to come to consensus on how to consistently interpret the memo the NOP issued on this topic, but they haven’t been able to come to a consistent interpretation.
Do you agree that this lack of consistency is a problem? What path is the NOP going to pursue to bring about consistency in how the three-year transition requirements are applied?
Tucker/NOP: Great question. I think the other tension I didn’t mention when Nate was talking – of this overarching framework of OFPA and nuance of site-specific decision making. Sometimes I am a little worried that the 3-year transition is framed as a waiting period when really it has to do with the application of prohibited substances. I think agreeing on what the problem is and what is going to be the focus moving ahead. The lawsuit on hydroponics was resolved now and provides more data for that issue. What do we want to work on here? What problem do we want to address here? How we view the 3-year transition – we all need to go back to what that means. The difference between certifiers – we need to look at those – it is not a waiting period – it is because of application of prohibited substances. There are some certifiers that have decided not to certify certain operations, and I’ve heard feedback that some certifiers are getting push-back from their community that does not make them feel that they can certify those operations. I do not know what the exact next steps are. I think we are engaging with the organic community and that has value.
2:15 PM ET: Lunch Break (to reconvene at 3pm ET)
3:00 PM ET: National Institute of Food and Agriculture (NIFA), Update on Organic Research Priorities
Mat Ngouajio, Steve Smith, and Neerja Tyagi are all present from NIFA.
Organic research priorities collected by the Board are sent to NIFA for consideration. NIFA staff will report on the process.
Mat Ngouajio gives the presentation, with slides: NIFA is the extramural funding agency within the USDA. Relatively small, with 300-350 people within the agency; $1.8 billion budget, most going to land grant institutions. Pleased to see growth in organic industry: 31% increase in sales.
Observing increase in organic sector in Europe as well. Seeing growth in production and processing of organic products. Noticed the growth in the organic sector has not been accompanied by organic research and development in world economies.
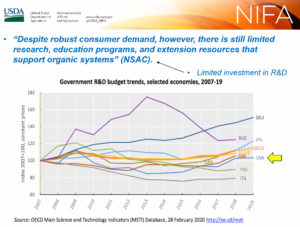
Two programs at NIFA: Organic Agriculture and Research and Extension Initiative (OREI) and Organic Transitions (ORG). Many other USDA programs support organic as well, such as NRCS, ERS.


Organic agriculture is facing challenges right now. We need to expand entire supply chain from field to table, and require many partnerships for this effort:
Industry action is needed to identify issues and their respective urgency. Congress has been responsive to calls for funding:
In 2021, Congress increased the budget to $25 million, increasing to $30 million next year, and $50 million the following year.
NIFA took industry priorities from all stakeholders and translate into Request for Applications (call for proposals). Then use a very strict panel review process to determine which proposals should go forward with weight given to science.
NOSB priorities become NIFA priorities. However, that input may be distilled to a single sentence or idea. Some applicants use NOSB priorities as a way to establish relevance of topic for funding.
The number of proposals, and those that were funded (about 1,700 projects have been proposed, 356 funded):
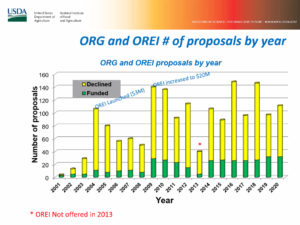
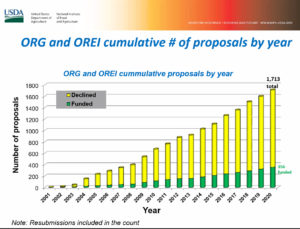
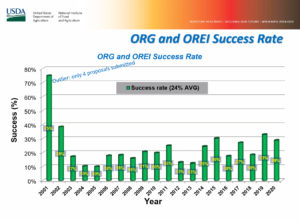
Of the 356 funded projects, average about 24% success rate. Most of the projects come from the West Coast.
Funding has improved for animal systems proposals, small and minority serving institutions, and for the Southern region overall. Seeds and breeding proposals are generally the most successful projects.
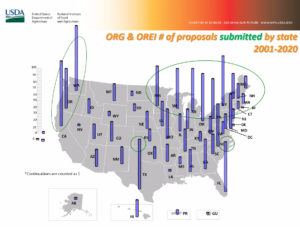
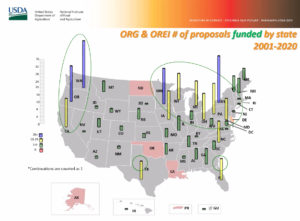
Increased OREI budget will be used to develop smart tools for farmers and processors (seeds and natural substances), for enforcement agents to improve organic integrity, to better serve people, and to ensure the materials are accessible.
NIFA contact information:

Questions from the NOSB for NIFA staff-persons:
Brian Cadwell: Worked on several OREI funded projects. What does “smart tools” mean in this context? Also, one of the projects he worked on via OREI was funded twice and dropped the third round. It’s a difficult adolescent period for long-term trials; they struggle from the 10th through 20th year, although they yield some of the most important data in that time period.
Steve Smith: We recognize the point about the long–term investment in these projects. Congressional appropriations only offer 5-year funding authority to NIFA. This puts long-term projects in competition with each other for funding. This is a valid point and not sure what the answer is. Open to suggestions about how to get around congressional guidelines.
Mat Ngouajio: Used to fund only for three years. Due to this complaint, that period is now five years. If the same projects are funded to the exclusion of other projects, there will be complaints. Additionally, once the project has a longer term data set, it makes the project more attractive for funding.
Smart tools could include a cultivator or new natural materials. Anything that removes barriers to farming.
Amy Bruch: So many facets to organic farming. Are there opportunities to collaborate with other countries?
Steve Smith: We do these collaborations that benefit the US. Gave example of crops not grown in the US. Support international collaborations when the primary investigator is a US official. Legislation that created OREI doesn’t allow incentives to collaborate in this way, but it does not prevent it.
Rick Greenwood: From the graph on success of the research. How is success measured? Is it expected outcome? The graph appears rigid, but research is not that way.
Steve Smith: Success refers to the success of the applicant, not the project. In hindsight some of the most successful projects were not clearly useful until after the research was completed. Others, like NASS, often look at this long-term success and utility.
Rick Greenwood: Some research universities measure success in Nobel prizes. I appreciate this problem.
Mat Ngouajio: We take significant risk in the projects because we will otherwise miss needed research. We cannot know how it will turn out in advance.
Kyla Smith: When projects are declined, is this due to lack of funding or does it reflect on the projects themselves?
Steve Smith: We use a ranking tool on the panel to help sort the priority of projects. Nothing lower than a medium priority can be funded. NIFA typically runs out of money before funding all high priority projects. Once NIFA is funded at $50 million, it is likely that more researchers will bring research projects to the process. If the number of applications jumps, the success rate overall will stay roughly the same.
Mat Ngouajio: If research is not focused on organic agriculture, it will not be funded. It must be performed on certified organic land.
Wood Turner: Chair of Materials Subcommittee. Learning this process is useful. Timing of materials process at NOSB is not in sync with the RFA process. Is there a way to optimize the process?
Mat Ngouajio: Biggest problem was to have a point person to work with. Michele Arsenault is now that contact. For timing, NIFA can always amend the RFA when the research priorities are posted. Observes it doesn’t change significantly from year to year. Be in touch with us and let us know when your research priorities are ready.
Steve Smith: When the NOP priorities are linked, from the old to the new, it helps NIFA follow the process.
Mat Ngouajio: We always put two years of NOSB priorities in the RFA.
Steve Ela: Glad to hear our recommendations are not just esoteric. Our list of research priorities continues to grow. Should we prioritize fewer ideas or is it okay to add more?
Steve Smith: NIFA wants to see the full list of NOSB priorities, as sometimes researchers already have ideas and can make a proposal. But your second audience is legislators. Those people are busy and they prefer to see a prioritized list of 5 to 10.
Mat Ngouajio: NIFA tries not to number priorities because each is equally important. You never know where you will get the brightest idea to solve problems. Do not prioritize for NIFA.
Steve Ela: Agrees prioritization is very difficult. Pleased to hear this. Thank you for supporting the NOSB.
3:50 PM ET: Livestock Subcommittee (LS), Sunset Review
Kim Huseman, Subcommittee Chair, facilitates the discussion of the LS substance review.
Calcium borogluconate

Mindee Jeffery presents information on this substance: Interesting substance in its history. One of NOSB’s questions: seeking clarity from the FDA on its classification of this material. Jared?
Jared Clark, National List manager, refers to the test of the final rule when this substance was added: was added, I think this paragraph from the preamble covers it (federal register .gov 83 FR 665599); noted there was some discrepancy from FDA and NL and it was addressed at that time.

Mindee Jeffery: Prevention is our best tool. Avoiding situations where this is needed for use. A couple of certifiers noted that the listing is redundant with electrolytes, but that it does not cause different decision making. One producer noted that continuing to list may eliminate confusion. One noted it is common and inexpensive. Producer group noted it is necessary to have a variety of treatments for this because they perform differently. Two vets commented this is an essential material for livestock treatment.
No questions from the Board.
Calcium propionate
(CAS # 4075-81-4) 205.603(a)(8) – For treatment of milk fever only.
Sue Baird: Used for treatment of milk fever, which is result of metabolic stress on or near freshening. Questioned if listing was necessary because of listing of electrolytes? Comments – heard that farmers need this. When a cow goes down with milk fever, it is past time for giving boluses or pastes or anything else. If a cow is down, this is needed. Internationally, this material is either listed directly, or may be listed as a method to prevent suffering of an animal. Internationally, it is accepted.
No questions from the Board.
Activated charcoal

Brian Caldwell: Considered a synthetic because of the activation process. 10 written comments in favor of it, none opposed, and a few that described it and its use but did not seem to endorse it at all. Several certifiers noted that only a small percentage of users listed this on their OSPs. It seems as if it is even being used for emergency situations it would be listed. I did not find any activated charcoal products on the OMRI list. The question I have is how necessary is activated charcoal for LS production. Hue Karreman noted this is an important tool. Several alternatives were mentioned, but nothing came out as a strong alternative; they were all sort of offhand. Perhaps I should point out that the emergency situation that requires activated charcoal is to take toxins or poisons out of an animal’s system.
Kyla Smith: Be careful when you are reading the public comments when certifiers are quantifying numbers. PCO does not currently have a way to tell the number of clients that are using this – when we list 7, that is the number of products that we have reviewed, but we could have 100s of producers that are using the product. Depending on certifier databases, it is really hard to pull out the number of producers that are actually using this product.
Chlorine materials

Nate Powell-Palm: Chlorine materials have been on the mind of the NOSB for many years as an integral disinfectant and sanitizer. Quite a few comments. Well considered comments on the role that chlorine materials have on dairies, and the need to make sure we have several different materials to avoid resistance buildup. Other comments that it’s one of the more economical materials out there, and that a lot of systems were built for chlorine use. A lot of the questions were looking at our sanitizer panel that we had last fall – when we look at these ecosystem of sanitizers – are there things we should be looking at to build in a more holistic system that leads to the suppression of food borne pathogens. Overall, comments were that this is an effective tool for livestock producers.
Asa Bradman: I think this comes up with all of the chlorine materials. The feeling is that people do not want to use them and would rather use something less toxic, but that they are essential given the mix of the materials that are available. The notion of a microbial ecology that fosters less pathogens is extremely difficult and did not see a practical way of doing that. Trying to use rotation and chlorine as a last-resort seems to be the goal.
Nate Powell-Palm: I think that was well-reflected in the comments. There is the goal, and there is the practical nature of not becoming known as an industry with food safety problems.
Amy Bruch: Comment from OMRI – it looks like they are reviewing the materials for use in milk houses as a handling-type mentality – can you provide a little more information on the crossover that we see?
Nate Powell-Palm: In every dairy, there is a small amount of “handling” that goes on – moving milk from cows to a bulk tank. Those surfaces that actually come into contact with the milk are going to be on-farm and fall under livestock. Dairies do not have to be certified has handlers. If we move to a creamery, there will be much of the same surfaces, but mostly dairy falls under the livestock listing.
Wood Turner: Want to pivot off what Asa was saying – looking at chlorine materials on 3 different committees. It is clear that we keep cycling on this idea that there aren’t a lot of options and there is a need to have rotations for sanitizers. Coming off the sanitizer panel and what to do next, it occurs to me that we need to be a bit more forceful in the research priorities about what we are looking for in chlorine materials. The research priorities really addressing this from a handling perspective and we should be addressing it from all perspectives.
Nate Powell-Palm: This is something that is going to take a whole community lift. As a livestock person and inspector of livestock, I hear you.
Kyla Smith: Sometimes within the NL items or certification in general we try to be consistent. I think this is an area where there are a lot of nuances of how these materials are used, especially within livestock and having to integrate other regulatory requirements – I do not want us to lose sight of that when we are trying for consistency – there are a lot of factors at play.
Steve Ela: Our community continues to ask us for more prioritization and decision-making on this, yet this is one of those topics where OFPA collides head on with FSMA. When Joelle was on the Board, she commented that every specific use as a specific use for these sanitizers. Her main profession was food safety, and I took her comment to heart.
Kaolin pectin

Kim Huseman: The tools in the toolbox, this material is used in specific situations, not all the time. It’s an alternative to activated charcoal.
Amy Bruch: Is there a limitation with availability with respect to synthetic versus non synthetic?
Kim Huseman: Don’t have the answer today.
Kyla Smith: Doesn’t recall seeing anything in comments on the topic.
Brian Caldwell: Gathered that act. Charcoal is a gentler product for acute, miler cases compared to kaolin pectin, which may be used for scours, etc. Is that accurate?
Kim Huseman: Having multiple tools in toolbox is important; does not know about livestock nutrition – didn’t have a specific answer.
Kyla Smith: Are you thinking about the language about nonamidated forms of pectic could be included…?
Kim Huseman: Yes, and then NOC had made the comment that there can be both synthetic and nonsynthetic forms, and if only the nonsynthetic forms were allowed, it wouldn’t need to be listed on the NL.
Nate Powell-Palm: Comment from a cultural perspective: ranchers have kept act. Charcoal for… pectin for routine minor microbial infections in calves.
Kim Huseman: Agreed, mentioned having the right tools in toolbox again for the cause.
Mineral oil

Sue Baird: Used for impactions normally in the ruminants’ third stomach. Happens in pregnant cows during colder months when consumes less water and eats lower quality forage. Mineral oil lubricates the impaction to pass through. Certifying agents material working group noted confusion about whether this material is delivered orally or rectally. NOC claimed it has been in use for a long time without being on NL.
TR listed alternatives, but yet mineral oils seem to be the best method. I always appreciate comments from vets and others who have a lot of knowledge of why or when minerals are used. Our favorite vet who used to be on NOSB said that it is indispensable to him. All positive comments received on whether or not it should be allowed. We did get a comment from Beyond Pesticides that said there was mis-correlation between FDA and NOSB and perhaps we should get clarification by adding a specific CAS# that should be allowed for this use, and yet they and we know that we cannot address annotations at this point.
Kyla Smith: I noticed that even within the comments there were inconsistencies from the certifiers about whether or not there are inconsistencies. I tried to seek clarification on that. From my understanding from the ACA Materials Working Group, most are allowing this both orally and rectally, but it was stated that it would be clearer if that was explicitly noted in some way in the listing. My understanding is that in the actual review process, certifiers are aligned.
Sue Baird: There were some comments or suggestions that we could use other types of oil, but it was noted that other types of oil break down in the gut and do not alleviate the issue. Other oils are not effective; mineral oil is effective.
Asa Bradman: I want to highlight that the other place we have mineral oils use is in horticultural oils for crops – two places where we have petroleum products used. I think in both cases these materials are important, but perhaps one of the challenges to think about when we think about other petroleum products.
Sue Baird: To complicated that further, it is listed twice in livestock – also at section 603(b)(7).
Nutritive Supplements

Nate Powell-Palm: What do we do to help acutely sick animals? Activated charcoal, mineral oil, nutritive supplements – means we are more able to address critically sick animals. As an inspector, I have seen the power of other injectable nutritive supplements. The comment that really stuck out for me said that if we didn’t have this material, we would go back to the dark ages. Comments were consistent that nutritive supplements are widely used and would be sorely missed and put farmers in a difficult position to keep the welfare of animals at the forefront.
No questions from the Board at this time.
Propylene glycol

Mindee Jeffrey: Updated TR has arrived and is posted. Derivation from petrochemical feedstock is also an exception that we are making with this substance. The same commenter noted that there are alternatives to the treatment and this wouldn’t be needed. Comment regarding intravenous dextrose administration as an alternative treatment for this. Another producer mentioned oral apple cider vinegar and molasses. They also commented that this substance is available in farm stores and considered very effective. A large animal vet noted that this material is the gold standard for ketosis.
No questions or comments from the Board at this time.
Sodium chlorite

Kim Huseman: A few comments written, no oral comments. There are producers that list this in their OSP. Less than 5 comments that said they support relisting. I do think it is important in the dairy industry. I think it was listing in both (a) and (b) to cover all of the basis, but I think as a work agenda item, we could look at cleaning this up and having it listed only once. We did ask stakeholders for alternatives and if there were changes in iodine availability, would that reduce the need? Those questions were not answered in comments. Hopefully we will generate more comments for the fall.
Kyla Smith: I did make a note that there was one comment that iodine availability will not affect the need for this. I will have to try to find the comment.
Asa Bradman: Beyond Pesticides submitted very thoughtful comments on chlorine materials and ASC; I’m curious what your response to those comments are? Perhaps we could have a discussion in the subcommittee regarding alternatives. I’m curious what your response to those comments are?
Kim Huseman: I’m not sure how to answer you.
Asa Bradman: I understand. Perhaps chlorine materials seem essential, but perhaps this is something we can discuss as we lead up to the sunset voting in the fall.
Kim Huseman: I did not look at the application here in conjunction with other chlorine materials. I looked at them very independently.
Asa Bradman: I will bring this up tomorrow when we talk about chlorine materials in Handling.
Nate Powell-Palm: One thing I think about with teat dips – dairies are so different – you can have a small dairy that is wickedly different – but they all need to make a safe food product. You have a really acute threat of food safety problems with milk, and how do you have as big of a toolbox as possible so that you can have this same federal rule apply to all dairies equally. I couldn’t speak to Handling, and I think it’s more nuanced in Handling. I think that is one issue with how you find alternatives.
Asa Bradman: My sense from people who use the material is that they see it as essential, but I was wondering if the committee agrees with that or could we have conversations about other materials that might be less toxic.
Sue Baird: There are very few teat dips that are for sanitary issues. We have teat dips that might be used for medicine when the teat it cracked – glycerin – if you have a milk product with high somatic cell counts, which is high bacteria in the milk, your milk is going to be dumped or goes into the cheese processing arm of distribution. There is so little chance of environmental issues – applied directly with very little – small farm where the farmer takes a rag and washes down the bag – you cannot allow those bacteria to impact that milk. That is my take on the thing.
Kyla Smith: The comment I was referring to was part of the OTA producer survey. That same respondent says that iodine is the preferred method, but ACS seems necessary for rotation. One certifier said they had several producers using this, but not commonly used. Does seem that it is something that is good to have in the back pocket for use.
Zinc sulfate

Brian Caldwell: For use in foot baths for hoof rot and other foot issues. Legitimate concerns about mining and disposal. But it does not have the same buildup in the soil as copper sulfate, which is the main alternative. Another issue with zinc sulfate vs. copper sulfate, sheep are really sensitive to copper toxicity; therefore, zinc sulfate would be the preferred alternative there. 10 written comments in favor of keeping it, two with annotation suggestions because of environmental draw backs. Our question was has zinc sulfate reduced the use of copper sulfate, and it does not seem as if it does. One certifier said they have 0 zinc sulfate users and almost 100 using copper sulfate. Again, only small numbers of farm plans included zinc sulfate. Question of whether or not it is necessary. There are some alternatives – peracetic acid and H2O2 – amazingly, vaccines have some efficacy against some of these foot organisms, although I think that’s more experimental. Formalin was the old fashioned approach, but it’s quite toxic. Copper sulfate is the main alternative and is mostly used. Feeling is that we are not going to do any annotations now, but that might be an avenue that we want to pursue in the future.
Kyla Smith: Zinc sulfate is relatively new – only allowed as of January 2019 – I do recall seeing a comment from a certifier that it was difficulty getting information from a manufacturer. I’m reaching out to staff on this. The other thing, there is no annotation regarding restriction on use of copper sulfate…such as have to use zinc sulfate prior to using copper sulfate.
5:00 PM ET: Resolution for the 2021 NOSB Spring Meeting: OLPP

On April 21, 2017, the National Organic Standards Board unanimously voted to pass a resolution requesting the implementation of the Organic Livestock and Poultry Practices (OLPP) rule.
The NOSB recognizes that consumers’ trust of the organic label and industry growth depends on the strength and consistent application of the organic regulations. NOSB has an integral rote in advising USDA in tis promulgation of these voluntary standards and strives to seek consensus among organic stakeholders in its recommendations to USDA and the secretary.
The Organic Livestock and Poultry Practices rule, finalized in 2017, and subsequently withdrawn in 2018, was based on an unanimous NOSB recommendation to USDA in 2011. The NOSB recommendation was the product of a decade of public NOSB meetings, lengthy discussions, public comment periods and consultation from organic producers, processors, consumers, and the veterinary and scientific community. Both the NOSB recommendation and the final rule issued by Secretary Vilsack in 2017 defined appropriate requirements for space, density and outdoor access in organic poultry production. Support for this rule has been expressed through public comment by major and growing organic brands. The rule is supported by organic producers, consumers, the industry, and the NOSB. The policy received over 120,000 supportive comments in the federal register representing over 99% of commenters. The NOSB stands by its 2011 recommendation to USDA on the organic livestock and poultry practices policy question.

Motion to accept the resolution made by Powell-Palm; Steve Ela facilitates the vote.
- Vote Yes – 13; No – 0; Absent – 1 (Logan – lost call)
5:14 PM ET: Adjourn Day One of the Spring 2021 NOSB meeting
Tuesday, April 20, 2021: Public Comments
[Public comments were exclusively via webinar this year due to response to the COVID-19 pandemic.]
Michelle Arsenault, NOSB Advisory Board Specialist, gave introductions and instructions for the public comments to come. Dr. Jennifer Tucker, NOP Deputy Administrator, then welcomed the NOSB and thanks commenters and audience. Special acknowledgement and thanks were given to the five NOSB members who were about to begin their last NOSB meeting.
NOSB members in attendance (all NOSB members we present at the beginning of the call except for Asa Bradman, who joined later):
Roll call:
Steve Ela (January 2017 – January 2022)
Nathan Powell-Palm (January 2020 – January 2025)
Amy Bruch (January 2021 – January 2026)
Logan Petrey (January 2021 – January 2026)
Asa Bradman (January 2017 – January 2022) joins late due to prior engagement (between 10-10:30am)
James R. “Rick” Greenwood (May 2018 – January 2023)
Wood Turner (January 2020 – January 2025)
Sue Baird (January 2017 – January 2022)
Carolyn Dimitri (January 2021 – January 2026)
Brian Caldwell (January 2021 – January 2026)
Kimberly Huseman (January 2020 – January 2025)
Gerard D’Amore (January 2020 – January 2025)
Mindee Jeffery (January 2020 – January 2025)
Kyla Smith (January 2021 – January 2026)
[NOTE: the current Scientist (Toxicology, Ecology, or Biochemistry) seat is vacant, meaning there are only 14 NOSB members at this time.]
PUBLIC COMMENTS
Noah Lakritz (California Certified Organic Farmers (CCOF))
Policy and outreach specialist for CCOF. Comment on substances: Copper sulfate, peracetic acid, tartaric acid. Asks NOSB to carefully review organic rice growers, especially in CA, as they rely on specific production methods (flood irrigation) that require copper sulfate to control tadpole shrimp. Peracetic acid use has doubled since this material was last reviewed among their members; supports re-listing. 55 CCOF members list tartaric acid, particularly to produce wine. Malic and citric acid are not replacements to tartaric acid.
Q: Would you make the same comment on copper sulfate as algicide?
A: Have heard from members it is essential for algicide, especially in flooded systems.
Phil LaRocca (LaRocca Vineyards & CCOF)
Chairman of Board for CCOF and organic wine grower. Encourages NOP and NOSB to solve problems of the past, especially to move on items the NOSB has passed. Peracetic acid is important in winery as a sanitizer; we had to use iodine in the past which required a rinse, defeating the purpose of sanitizing.
David Gould (FoodChain ID / Bioagricert)
FoodChain works to identify GMOs in food chain. Companies who distribute new genomes should be required to identify those details so that they can be tracked. This is a growing threat to the organic sector. Excluded methods: we should be focusing on new methods of genetic engineering so that we know how to track and test for them.
References IFOAM: Organics International position paper on Compatibility of Breeding Techniques in Organic Systems:
https://www.ifoam.bio/sites/default/files/2020-03/Breeding_position_paper_v01_web_0.pdf
Eugenio Giraldo (NuOrganics LLC)
Comment on ammonia extracts. Nutrient recovery for organics agriculture. Comments on Tech Evaluation Report (TER) for ammonia extracts. The TER focuses on pure synthetic ammonia extracts, but the definition of ammonia extracts includes a wider number of products some of which are already approved by OMRI. TER fails to address benefits of low carbon liquid nitrogen fertilizers. High nitrogen and little phosphorus allow more precise application, helps environment.
Q (Ela): How would we prevent the overuse of ammonia extracts if they are allowed?
A: In favor of annotation of percent of total nitrogen; precedent of chilean nitrate.
Q (Bruch): From your point of view, when you look at soil and non-synthetic vs synthetic ammonia is applied, is there a difference in soil microbiome? Or is there any way to test?
A: Yes, you can test using infared spectrometry. Liquid fertilizers are derived from waste materials, and when it’s a natural ammonia there is always a carbon footprint of the waste material the fertilizer is derived from.
Hamsa Shadaksharappa (RenewTrient I LLC /Organic Fertilizer production)
RenewTrient produces ammonia extract from chicken manure. In support of naturally produced ammonia extracts; there are many products that were specifically developed for organic standards and are listed on OMRI. Issue of “transportable fertilizer” which straight manure cannot do. Supports application limits. Outright banning products is problematic. Should remain: products that recover ammonia from animal manures (should retain some level of carbon and natural material).
Q: Support an application limit?
A: If products have nitrogen content higher than 10-15%, it is prudent to have application limit. Greater than 12% especially would be happy to consider.
Q: What are the max % nitrogen of the products you offer?
A: 8% product approved by OMRI, that has a 7% limit by CDFA. We have capability to make higher % nitrogen products (between 12-15%) and that’s very desired in the market.
Jason Ellsworth (Wilbur-Ellis Company)
Ammonia extracts allow farmers to be efficient; problematic that animal-based nutrients have to be transported/shipped to satisfy plant needs. Extracting nutrients from waste ensures valuable nutrients get to a crop more efficiently. Manure-derived products complement but do not replace other nutrient materials.
Q (Ela): Other products contain ammonia, but extracts seem to be pure ammonia. In favor of listing the % of ammonia in product to discern the difference?
A: Not in favor of % of ammonia in the product, more in favor of limiting to a % of application, crop-dependent. Certifiers are capable of tracking that; they have the crop-specific knowledge.
Alison Watkins (International Food Additives Council (IFAC))
Handling comment. Supports relisting of carrageenan, agar agar, silicon dioxide, cellulose, etc. Carrageenan’s functional properties cannot be replaced; multiple additives would be needed for the same effect. Carrageenan required for many vegan and vegetarian products.
Agar agar is less temperature sensitive and has other important features, popular for new food products. Gel source for vegan and vegetarians.
No organic cellulose; no alternative.
Lynn Coody (Organic Produce Wholesalers Coalition)
OPWC wants NOSB to receive technical assistance, including research and technical writing (to help things move through more swiftly). In support of paper-based planting aids—concern about subcommittee language however (see written comments). Biodegradable bio-based mulch film (BBMF) comment: supports incremental improvement toward higher % of bio-based content. The final sentence of the recommend annotation creates a loophole for BBMF. Supports work on ammonia extracts; need clear ruling on this material for clarity for everyone.
Adam Seitz (Quality Assurance International)
Senior reviewer and policy specialist for QAI. Supports NOSB to have additional paid resources to do their work, but NOSB must remain independent. Agar agar and carrageenan: are these materials appropriately classified? Re-charge materials must be on National List (ion exchange filtration). Resins for ion exchange should not require inclusion on the National List.
More clarity needed on categories for what should and should not be on the National List.
There was a $7.5 million appropriation to support marketing for GMOs! We need this money for the organic program instead.
Jennifer Berkebile (PCO)
Supports work on NOSB workload and technical help to support NOSB work, including legal support to clarify authority. Support hiring of help for NOSB. Supports paper production aids as written. Support for BBMF products currently in the marketplace; the proposed revision to the BBMF allows 80% bio-based and PCO supports this if there are products on the market or soon-to-be created that will meet this standard. Part of the BBMF revision is confusing (the term “available” needs to be clarified).
Patty Lovera (Organic Farmers Association)
Paper–based planting aids are critical to small farms; support proposal and definition change. Ammonia extracts: majority of those who answered their 2021 survey said they would not use ammonia extracts. Urge NOSB to gather more input about farmer impacts with ammonia extracts. Continue to look for enforcement in fraud. There is a pressure to increase yield due to fraud because land is too expensive.
Priscilla Iskandar (POPCORNOPOLIS.COM)
Skipped/not here
Aimee Simpson (PCC Community Markets, Director of Advocacy & Product Sustainability)
Certified organic retailer. Climate change is a top priority, soil-based and pasture-based livestock are important for climate mitigation. NOP should set climate change as programmatic and NOSB priority; this includes enforcement of existing standards but also assuring better alignment with climate mitigation in ongoing regulatory work. Heavy metals contamination is within the scope of OFPA, and there should be better testing and manuring practices to ensure protection. Kasugamycin; majority of organic producers said there are organic systems management methods to prevent the spread. Encourage the NOSB to reject antibiotics in organic; threat to organic label to allow antibiotics in pears and apples.
Q (Bruch): Carrageenan and connections with Irish moss in written comments; is Irish moss a substitute?
A: Whole Irish moss is an important ingredient in cultural heritage and cooking. There is a difference in carrageenan and Irish moss and as an entity—health concerns and use as an extract may be different and the NOSB is making a connection that hasn’t been evaluated properly. We don’t allow carrageenan in our products due to the health effects.
Encourage more work as there are concerns about certified organic seafood.
Amalie Lipstreu (Policy Diractor, Ohio Ecological Food and Farm Association)
Organic agriculture is more resilient and should be part of climate mitigation and adaptation strategy. There is no other agricultural system that is as comprehensive for climate mitigation. USDA has a request for comment (due April 29) re: climate change; it’s important for the industry to respond including the NOP and NOSB.
Mark Kastel (Director, OrganicEye)
Organic market is a victim of our own success; multi-million-dollar certifiers certifying multi-billion-dollar corporations. The infrastructure facilitating the “busywork” of inspections allows for cheating in the paperwork. A broad-based task force should investigate alternatives to the current system of inspection and certification. Example, the $5,000 exemption for small producers has been frozen, not accounting for inflation. Many reports of drive-by inspections and the burden on honest participants is a problem, while high-risk businesses are getting token non-enforcement letters and poor inspections.
Q (Baird): Risk-based inspections remark is appealing; how would you determine which inspectors were more qualified? How would you change inspections?
A: Too many certifiers bid-out their inspections to the lowest cost, often to young people with no production experience. If we were to do fewer inspections every year, we could enlist the more highly qualified inspectors for the higher risk businesses. Should dial it down so there is less busy work—inspect based on past performance rather than every year (though be subject to random inspections at any time).
Julia Barton (Ohio Ecological Food and Farm Association)
Containers work agenda item is on hold, but this should get back on active work agenda.
Timing of meetings: OEFFA wants different meeting timing to allow better stakeholder participation. Currently not inclusive to producers.
NOSB research staff: Support the NOSB getting help; we want the NOSB to be well-equipped. NOSB members seek or get unsolicited research from the community all the time.
Comment (Bradman): Agree we need more work on the container issue.
Michael Sligh (Alliance for Organic Integrity, (AOI))
Former NOSB member. Focus on strengthening fraud prevention. We urge a focus on basic organic principles being carried out in consistent and risk-based approach; including biodiversity, soil health–based, real access to the outdoors, good communication between product supply chains and inspectors, etc. Focus on climate–friendly practices to remain strong and relevant.
Q (Bradman): Backlog of issues I think need to be addressed: OOL, List 4, and OLPP. Are there any others you are concerned about?
A: [not available]
Harold Austin (Former NOSB member)
Chair for Northwest Horticultural Council Subcommittee. Support relisting materials for handling and crops subcommittee. List 3 inerts are needed; very important for tree fruit and berry growers. Under prohibited materials for calcium chloride, but it’s allowed to treat physiological uptake disorders. The need for foliar calcium has never been more important for tree fruit growers. Chlorine materials, ozone, and peracetic acid support. Support relisting of sanitizers.
Q (Bruch): Notes comment in favor of kasugamycin in written comments; wanted to hear more of that perspective.
A: [in chat] Kasugamycin: Yes, we can farm large scale organics without the use of this material, except for certain varieties of pears and the Pink Lady apple. However, certain geographic locations in the U.S.A. have extremely difficult times and would benefit significantly in the control of fire blight if this material was allowed. This material would not work on an emergency basis because to apply it appropriately you would need to follow one of the blight models and apply it prior to an infection period.
Russell Taylor (Humic Products Trade Association)
The definition of ammonia extracts is too vague and could impact humic acid use. Hydroxide use with humic acid; all humic acids are alkaline soluble. There are flaws in the petition; the dose makes the poison. Humic acid applied to at small rates equates to a drop per square foot. Would like the clarification that humic acid extracts are not included under this rule.
Q (Bradman): How would you change the definition?
A: Just to make it explicit that the ammonia included with humic acids is incidental and that humic acids are still allowable.
Alan Lewis (Natural Grocers)
All Natural Grocers stores are certified organic handlers. We see uncertified grocers stating that they are certified organic and use the label. USDA certification should be a pre-requisite to display the seal. Leading organic retailer; we see a rise in skepticism among consumers; questions about soil-less production, fraud, community sovereignty, animal welfare, etc. OFPA is silent on many important issues. Third-party labels like Real Organic Project and Regenerative Organic Certification are broadening what consumers are aware of. Foundations of the organic program are being removed more and more: concentrated feeding, Origin of Livestock, group certification, etc. Provide an opportunity for critics to criticize the organic label. Should be skeptical for allowing more compromise: holes in the tent, not a bigger tent.
Q (Wood): Are you getting requests from consumers that have the ROC/ROP standard?
A: Being asked about all the problems in organic by consumers; occasionally get requests to bring in ROP labeled products. Consumers concerned about industrial organic.
Michael Crotser (Organic Valley/CROPP Cooperative)
Certification manager at CROPP. Comment on sanitizer compliance review and dairy industry challenges. Review of sanitizers of inbound container tanks is needed. A lot of time and money spent re-sanitizing containers for organic milk. Although water rinses are allowed, the chain of control is broken when these washes happen and they introduce bacteria which make milk handling more unsafe. Limited location for re-sanitization events. Solution: certifiers must expand role in review of sanitizers used for tanks. All wash tags should include last sanitizer and water rinses should be eliminated. There is also a need for new sanitizer chemistries.
Q (Bradman): Specific chemistries in pipeline that are important and may be appropriate for National List.
A: Chloride materials, peracetic acid are the two major groups available to organic handlers now. There are some products already on the market that should be considered for the organic marketplace.
Comment: Appreciated comments on CACs inspector pools in written comments.
David Epstein (Northwest Horticultural Council)
Represents growers, shippers, and packers for apples, cherries, etc. Concerned proposed draft framework about appropriateness of certain materials in organic is not in compliance with sunset review process. Sanitizer issue best approached as research priority. List 3 Inerts are important; review needs to allow use in the meantime. Pheromone disruption is an ecologically sound method of controlling pests. Supports adding kasugamycin to National List; supports limiting number of applications to manage antibiotic resistance.
Q (Bruch): Peracetic acid in written comments show some use in controlling fire blight. What are pros and cons (and comparison) of peracetic acid and kasugamycin?
A: The statements we make come from the input of growers. Some of the products available now are not as effective as they need to be. Apple and pear production: growers would like to use all these products. Fire blight can destroy a business and kasugamycin could cure this. Comes down to variety differences; it probably wouldn’t be used across the board but bad weather situations increase risk.
Q (Jeffery): Other antibiotics went off National List; what’s changed since then?
A: Growers doing more pruning out since then; much higher cost in labor. It depends on the planting system.
Jill Smith (Executive Director, Western Organic Dairy Producers Alliance (WODPA))
Origin of Livestock (OOL) rulemaking: producers unanimously support strong, enforceable, and fast rule. Supports discussion on human capital management; we’ve faced inspectors who don’t know about dairies. Concerned with greater costs with cost share being cut back; too much labor in recordkeeping. Supports policies protecting organic integrity. See written comments on livestock materials. Chlorine and other sanitizers are also essential for dairy to provide safe food for all.
Comment (Bradman): Appreciate comment over OOL law; we all need to keep working on it.
Kathy Park Price (Garden Train (school gardens consortium))
SKIPPED
Christie Badger (Consultant with National Organic Coalition)
Paper pots: request NOSB acknowledges listing has issues that need to be addressed; missing requirement for continuous improvement.
Q (Bradman): Everyone seems to want plastic in organic, whether polyethylene or biodegradable. Paper pots we are allowing up to 20% non-biodegradable; how do we tackle this issue?
A: We don’t completely move away from petroleum-based products just because we go toward a bio-based mulch. We don’t get away from “plastic culture” in organics just by changing the type of plastics used. Container production has ground cover down for years on end. Paper pots vs. biodegradable film: paper pots usage is a much smaller use (mulch film is thousands of acres covered). With the plastic change we have a chance of removing it—the biodegradable mulch we do not. We don’t understand what goes on under BBMF. We don’t have enough research.
Abby Youngblood (Executive Director, National Organic Coalition)
[NOTE: this comment was provided in full by the presenter.]
Good afternoon. I’m Abby Youngblood, executive director at the National Organic Coalition. I would like to welcome the new NOSB members and thank all of you for your work.
The National Organic Coalition or NOC is an alliance of organizations and companies that works to advance organic and protect the integrity of the organic program. We see an important opportunity at this moment in time to work with new leaders at USDA and in Congress to get organic back on track.
Our top priorities with the new administration include reinstating the Organic Livestock and Poultry Practices rule, finalizing Origin of Livestock and Strengthening Organic Enforcement proposed rules, fixing the funding shortfall with organic certification cost share, building a more equitable and diverse food and agriculture system, and demonstrating to Congress the role organic agriculture can play as a climate change solution.
We see an important role for the NOSB on this last topic. NOC is requesting that the NOSB create a work agenda item focused on climate change mitigation and adaptation and enforcement of soil-health provisions in the organic regulations. We are asking the Board to say no to ammonia extract and to recommend a detailed restriction on the use of highly soluble sources of nitrogen. Over reliance on highly soluble sources of fertility can short circuit soil-building practices and violates the foundational “feed the soil” principle in organic.
On a different topic, an ongoing and egregious area of inconsistency in organic is how the three-year transition requirement is applied. NOC conducted a survey in in 2020 with the Accredited Certifiers Association and the Organic Farmers Association that demonstrated the high level of inconsistency on three-year transition requirements. But the NOP has not taken any action to address the problem. We are asking that the NOSB actively engage on this issue and urge the NOP to provide clarity so all certifiers and organic operations are held to the same standard.
On excluded methods, the organic community and NOSB have been clear in our opposition to genetic engineering in organic. We need clarity on which methods are allowed and which are prohibited under the organic regulations. Failure to complete work on the TBD methods and to codify NOSB recommendations will negatively impact organic plant breeders and the organic seed industry, who need certainty to advance plant breeding efforts.
Finally, I have a comment on ion exchange filtration. The NOSB should use OFPA as a guide and review and recommend which nonagricultural substances used in the ion exchange process must be present on the National List. We urge the NOSB not to give the NOP the final decision, but to remain true to the authority granted in OFPA to make recommendations to the NOP on National List materials.
Thank you for considering my comments.
Q (Ela): Do you think we should review each resin separately?
A: Defer to Terry Shistar.
Jay Feldman (Beyond Pesticides)
Every NOSB decision is important to the strength of organic; if we lose public trust, we lose the market. Chemically intensive marketplace has helped drive the organic market; the organic law requires NOSB to consider cradle to grave of substances. The USDA cannot ignore NOSB recommendations. Sunset should default to the removal of substances. Changing previous NOSB decisions without new science undermines integrity. Weaking BBMF will undermine attempts to protect soil and food.
Q (Bradman): BBMF: see how many organic farms use plastic to cover the ground, basically petroleum-based additive even if it’s just a barrier. Many in organic community are demanding a biodegradable mulch. There don’t seem to be many options—can you comment on it?
A: Standard used previously: to guide whether what we were doing was incentivize the market to moving toward continuous improvement (total market improvement). Wrapping of plastic has been allowed by lack of attention to mulching systems; need to move toward continuous improvement. BBMF fear is that we are intentionally leaving material in the soil; can’t even remove to recycle/re-use. If we introduce something that slows down where we want to be, there should be a pause in allowing that practice/substance.
Terry Shistar (Board, Beyond Pesticides)
Review of their organic comments. The world needs more organic; organic is not just an alternative food. We need to prevent ecological disaster. Reject kasugamycin (and antibiotics in general); antibiotic resistance is serious. Planting aids should not be made from virgin paper. Microplastics are serious—get plastic out of organic. Prohibit ammonia extracts. Ion exchange: these substances must be reviewed by NOSB. Products created through ion exchange are synthetic; labels should include all ingredients used. Heavy metals are in infant foods and consumers expect organic foods to be safe. More work is needed on keeping food safe.
Alice Runde (National Organic Coalition)
Coalition Manager. Racial equity: access to organic certification and marketplace has not been equal across racial groups. Should consider impact of decisions on BIPOC farmers. NOSB should prioritize research into issues faced by farmers of color and under-served communities; work to identify and translate materials.
Modernizing regulatory review executive order.
NOC supports additional funding to support NOSB, including assisting NOSB members with research, etc.
Research priority on heavy metals in baby food is needed.
Jo Ann Baumgartner (Executive Director, Wild Farm Alliance)
Submitted draft Native Ecosystems guidance to jump-start process. Protecting native ecosystems slows climate change, something Biden administration and consumers care deeply about. This regulatory issue is an international issue; NOP is charged with ensuring standards are consistent throughout, but it is undermining consumer confidence to allow destruction.
The guidance outlines how to determine what is a native ecosystem. Also created a toolkit to help certifiers and others to better understand the available tools. WFA is here to help.
Doug Currier (Technical Dir. Organic Materials Review Institute (OMRI))
Ammonia extract and List 3 Inerts. Liquid fertilizers that meet the petitions definition are currently on the OMRI list. Refer members of NOSB to specific restriction language for these products (see written comments). List 3 Inerts: 18 pheromone products exist on OMRI lists. There are a limited number of List 3 Inerts used in these products, so the NOSB could consider separately listing these inerts on the National List.
Q (Ela): Some products that have greater than 3% ammonia are already approved; are their suitable alternatives to those products if we were to accept the petition. Products higher than 3% ammonia at this point
A: The sodium nitrate that is already listed; products that are less than 3% nitrogen. Some alternatives but they vary in solubility and form.
Q (Caldwell): If extracts were prohibited, would it also prohibit fish emulsion and other fertilizers that contain some ammonia?
A: Depends on how it’s written at 602; there could be a risk. The definition of ammonia extracts is so broad it might unintentionally prohibit products of less concern.
Karen Howard (CEO, Organic & Natural Health Association)
Specialized trade association for nutrition supplements. They test for glyphosate residues. Education focuses on relationship between environment and human health. Degraded organic standards is a concern for their industry. Educate about purchasing organic food helps sustainability, human health, etc.
Michael Menes (True Organic Products)
Organic fertilizer manufacturer. For prohibition; none of it should be allowed because it’s not compatible with organic principles. Clarifying definition of ammonia extract: a fertilizer produced using a range of methods where the output contains ammonia (NH3) and/or ammonium (NH4+) that has been intentionally produced through a biological or physical process, captured in liquid form, concentrated and/or extracted, packaged for application in a crop system.
Q (Ela): Would there be a limit you would support if it was allowed? Are there alternatives?
A: Only for a complete prohibition. Other materials available address these needs.
Ramy Colfer (True Organic Products)
Research agronomist and previously worked with Earthbound Farm. Organic farming normally built on slow building of organic fertility; require time. Building up natural enemies of plants requires biological systems to be functioning properly. Allowing mineral-based ammonia is nothing like what would be found in nature. Organic farming has been decades ahead of regenerative agriculture practices. Ammonia extracts would push organic toward conventional farming; should be prevented.
Q: What’s the difference between ammonia extracts and other [fertilizers]?
A: Not in mineral form; requires soil biology to break them down into usable nitrogen. Ammonia extracts skip this process.
Q: Should sodium nitrate also be prohibited?
A: Yes, it should be prohibited. We never used it at Earthbound and grew thousands of acres of leafy veggies. It’s a crutch that was grandfathered in.
Ehsan Toosi (True Organic Products)
Difference in organic and conventional is difference in nutrient cycling. Supplying nitrogen through synthetic fertilizers bypasses fundamentals of organic farming, specifically soil health. For prohibiting ammonia extracts.
Zea Sonnabend (Fruitilicious Farm)
Former NOSB member. See written comments; also wants to speak about inerts. The List 3 Inerts: it would be ideal to put them on the National List individually because there are so few of them. There are previous petitions that have not been fully dealt with that can bring this issue forward. NPEs issue can be advanced to a proposal and removed from the National List. NOSB needs to keep the pressure on in regards to inerts policy to help the department and interface with the public.
Supports ammonia extracts petition. Also authored the kasugamycin petition. The Technical Review gives accurate research on the environmental/health effects of kasugamycin because it’s not used in those applications. The comments coming in are more about resistance transfer, but that relates to streptomycin research and other antibiotics that are more widely used.
Q (Ela/Greenwood): TR noted it was not a good idea to apply it after a certain amount of time after animal products were applied.
A: It’s only used during the bloom period, which is only a few weeks. In CA we have extensive “rat tail” bloom because of the weather. This is why other practices don’t work as well in that region (especially pears).
Q (Greenwood): Change in soil microflora after Kasugamycin has been used and it is another aminoglycide, similar to streptomycin. What Steve is referring to is probably animal grazing. We should use the precautionary principle; better to have the research before we move forward because it could be we find there was an issue three years down the line (as an example).
A: Not enough research on how long the microflora change lasts and whether there are practices that mitigate it.
Angela Wartes-Kahl (Independent Organic Services, Inc.)
Consultant and organic inspector. Human capital proposal comment: the apprenticeship proposal outlines components of a good apprenticeship program. International Organic Inspectors Association (IOIA) is the professional organization of organic inspectors and offers training and networking world-wide for crop, livestock, and processing inspectors. Intend to launch first apprenticeship program though IOIA, focusing on organic livestock. Highlights that IOIAs reach is international.
Need to develop best practices for issues like pay scale and recruitment. Inspectors have been stakeholder in industry since the beginning and now we need to strengthen the career path.
Q (Baird): How can we use industry funding/grants without conflicts of interest?
A: Asked for a white paper on conflict of interest to see if brands paying for scholarship apprentice fund, and the white paper found it wouldn’t be an issue.
Q (Bruch): How do you work with different organic standards internationally.
A: Training inspectors all over the world. International trainings are all based on the NOP; all for countries that would be importing to US under the certificate. Person right off the street with no experience does not have the basic experience to enter the apprenticeship. There is an extensive application process for IOIA, not pay-to-play.
Jake Evans (True Organic Products, Inc.)
Mimic conventional marketplace where profit determines decision-making versus preserving organic standards. Sodium nitrate is already available but if you apply to much, you will kill your soil. That’s not true for ammonia extracts; you can use a lot more without internalized restrictions. Organic industry should not pay for conventional industry waste product. Asks for prohibition on ammonia extracts.
Q (Jeffery): Do you think this would hurt your own profits as an organic fertilizer producer?
A: Could have sold ammonia extracts for 10 years and choose not to. I hope the NOSB does not force my business to get into ammonia extracts.
Jessica Shade (Director of science programs, The Organic Center)
Research priorities: happy about evaluation of BBMF, whole–farm assessment of farming impact choices, no-till, relationships between biodiversity and pathogen practices, etc. The OC is actively involved in research on these issues and NOSB is very helpful for securing funding.
Suggestion for additions to research priorities based on feedback: benefits and risks of livestock, nutritional value of organic animal products, protection of organic farmers from chemical contamination, and comparison of pesticide/antibiotic/growth hormone residues in organic vs. conventional. Would like research for celery powder alternatives.
Also submitted comments on ammonia extracts.
Q (Ela): Comments on ammonia extracts?
A: We can’t know how the extract will interact with soil until research is done on each specific product. We can make broad assumptions based on research in synthetic ammonia products; they alter things like soil microbial communities, nitrogen mineralization varies which affects leaching and other factors, etc. Studies need to be conducted in an organic setting when they are done. The extraction process does change carbon sequestration process in the soil (organic soil amendments had the biggest impact on carbon sequestration so that part of the process could be impacted).
Megan DeBates (Organic Trade Association)
Vilsack has laid out agenda; advancing organic agriculture must be essential to the program’s move to help mitigate climate change. The NOP needs to be strong to advance these goals; not enough priority put toward standards development and regulatory changes were never intended to have to go through Congress (mentions congressional asks to push forward with Origin of Livestock). The NOSB was created to address standards issues, their recommendations should be taken seriously, and their recommendations should be implemented.
Laura Batcha (CEO of Organic Trade Association)
Organic Livestock and Poultry Practices Rule (OLPP) update: the underlying policy choices by the USDA going forward. In 2009-2010 the NOSB worked on a recommendation that passed in 2011. There is broad support for policy recommendations NOSB brought forward and the final world, including outdoor access requirements and restrictions on alterations. Concerning to hear Vilsack say he felt the department needed to start from scratch on OLPP. This is a mistake; there is already a lot of work done from the NOSB and community. Push to reinstate final rule from 2017.
Mary Capehart (CROPP Cooperative, Certification Senior Specialist)
Human capital management comments. There is a critical shortage of qualified organic inspectors. Served by 30 different certifiers and constantly receive feedback; frustrating that inspectors do not have farming background; inspectors have concerns about liability from performing on-farm audits. Regulations do not include mandatory requirements for qualifications. ACA brought forward qualifications requirements.
Q (Baird): Would be happy to share my experience with new inspectors, but not for free. How would you feel about funding some kind of mentorship?
A: Organic Valley wants to be part of the solution.
Dragan Macura (AgroThrive, Inc.)
Makes dry and liquid fertilizers. Address fraud issue for ammonia extract material: nitrogen is the most sought-after nutrient by organic farmers. Fraudsters just add ammonia to other existing fertilizers. Ammonium extract from natural sources is not a problem, but it’s indistinguishable from synthetic sources. Add ammonia extract to 206.602 list or at a minimum put a hold on it until testing methods are developed.
Tom Buman (CEO of Precision Conservation LLC)
Comment on ammonia extracts. Concern about definition because it’s not coming from academic researchers. The definition is concerning and may eliminate appropriate products. Concern for fraud: this argument should be approached cautiously; it could be made with any organic product. All parties should work together to create best practices. There is an agronomic need for new nitrogen products. Manuring and compost will always be present, but new products are a way to balance nutrient needs while protecting water quality from over-application.
Q (Ela): If we don’t prohibit them, would you be in favor of an annotation to certain % of nitrogen?
A: Yes, that is a consideration. Need to continue to use soil-heath practices. Not enough research/need more information to determine a specific number for limits.
Diana Kobus (Executive Director, PCO)
Human capital management. Qualified inspectors are keystones in upholding organic integrity. The NOSB’s proposal is not directive enough to address this issue. Inspectors require high degree of knowledge similar to that required to be an organic farmer; inspectors should similarly be recognized for their high levels of skill required. There should be standard training of inspectors and other staff. We need resources brought to this issue in a way that is unified and with the right urgency. This work needs to come from NOP.
Q: The unification of this program should be NOP?
A: Yes, NOP should set standards and enforcement.
Linley Dixon (Real Organic Project)
[These comments were provided in full by the commenter.]
I’m Linley Dixon, co-director of the Real Organic Project. The Real Organic Project is a collection of now 850 farms and growing quickly. These farmers have come together behind the fundamental organic principle of soil health.
I run a certified organic vegetable farm in Southwest Colorado specializing in soil-grown tomatoes, greens, peppers, cucumbers, and herbs. All products that are increasingly hydroponic and organic. This comment is going to seem really rudimentary, but sadly we find ourselves here. Our farm, like many others, prides ourselves in our composting. We cycle local veggie scraps and landscaping waste into compost that is then added to our soils. No liquid feeding. No mining for added inputs. Please keep this in mind when making decisions – that organic farming was founded on the basic principle of cycling farm generated organic matter and local organic waste back into the soil. Many certifiers do enforce the soil health standards in OFPA. Many do not.
Remember that the lowest standard allowed becomes the standard. When poultry porches were allowed as “outdoor access,” now over 75% of the organic eggs are produced in buildings with porches. When the standards are weak, other labels rise. Pastured “non-GMO” eggs are everywhere – outside of USDA organic. Lets keep organic strong.
Remember that “A big tent” means the cheapest system wins. I directly compete with certified organic growers that simply fertigate with unsustainable off farm inputs at every watering. Many use hydrolyzed soy originating from conventional GMO soybeans – soybeans that are synthetically fertilized with multiple herbicide passes, and then synthetic acids are used to “hydrolyze” them. These conventional soybeans are grown just for the organic hydroponic market. We could save a lot of energy by just certifying the conventional GMO soybeans!
Real Organic farming requires that you add enough locally generated organic matter to the soil to retain water and release nutrients slowly over time. So there is minimal nutrient leaching. This is how we filter and replenish water in our local aquifers, and how we sequester carbon in our farming practices. If organic continues to allow hydroponic and confinement livestock production, organic will become irrelevant when it comes to mitigating climate change. The “regenerative” glyphosate farmers will get the carbon credits.
While CAFOs and hydroponic producers shop for the right certifier to approve them, many of us shop for the ACA with integrity. Please use your esteemed position as a National Organic Standards Board member to stand on the side of Real Organic. Publicly fight for Real Organic and remind the world why organic is always the best choice. Lets work to incentivize the market to move toward our shared principles. Thank you for volunteering your time.
A: Comments from conversation with Miles McEvoy.
Q (Bradman): We do need standards for container systems. Nitrogen also needs to be discussed by community; most organic systems are importing conventional manure. A lot of challenges and hope that we can take out the conventional sources. The discussion of ammonia extracts could prompt discussion on other sources of nitrogen.
A: Might as well allow synthetic ammonia if we are going to allow hydrolyzed soybean that was grown with synthetic nitrogen. Some ACAs are not certifying hydroponics which uses these highly soluble fertilizers.
Patrick Kerrigan (Organic Consumers Association)
There is an erosion in consumer trust because of a lack of organic enforcement in organic livestock and poultry especially. There is widespread greenwashing of regenerative now. The spirit of organic should be regenerative but consumers remain confused. Organic consumers are fed up from ongoing erosion of organic standards while they are paying a premium price. Organic producers creating their own add-on labels which consumers thought the NOP was already enforcing. There needs to be more dedication to racial equity.
Laura Colligan (Dirtrich Farm)
Consumers may define organic by what it isn’t, but organic farmers use the mantra “feed the soil, not the plant.” Hydroponic operations do not have a relationship with the soil. As consumers learn how much of their organic produce is grown hydroponically there will be a backlash against the label. I disagree with hydroponics in organic label, but there needs to be standards if it’s allowed to remain. Many activities and materials used in hydroponics which have not been reviewed for use in organic like lighting, long-term landscape fabric, etc. It’s important that all organic producers are on a level playing field.
Martin Burger (Environmental Scientist, California Department of Food and Agriculture)
Comment on ammonia extracts. CDFA does not regulate application levels but if there is an annotation is could easily be implemented. Concern about leaching organic soil carbon: could not find evidence of this concern. Additions of nitrogen generally lead to increases in soil carbon when compared to adding no nitrogen at all. Poor soil management practices can lead to declines in soil organic carbon.
Q (Jeffery): Elaborate on the isotope tracking.
A: Nitrogen isotope analysis is based on samples taken from finished products; we can analyze for nitrogen isotope ratio and that number does not change. So it can be tracked/identified on that level. You could track fraud this way.
Q: Does it matter what form of nitrogen you are adding, re: effect of soil carbon?
A: The research has not been conducted and it matters which system you are talking about.
John Foster (Wolf & Associates)
Not representing clients to NOSB process. There is a need for additional support for NOSB members. NOP has provided better Technical Reviews and support staff which has served the community well. Regulatory comparisons, alternative practices, draft proposals/regulatory language writing, and economic analysis, etc. would all be useful.
Dan Hazen (Perfect Blend, LLC)
Fertilizer producer. Ammonia extracts Technical Review does not track history with scientific literature. The TR compares them to other materials with assumptions that are not accurate (conflating synthetic and natural). Ammonium stripping technologies are different than ammonium concentrate technologies (no extra processing steps). There is no evidence of fraud existing in the marketplace; should not create policy from fear. Trade disputes comment.
Heather Spalding (Deputy Director, Maine Organic Farmers and Gardeners Association)
See written comments. Actions would like to see: restore cost share, reinstate Organic Livestock and Poultry Practices Rule, issue Origin of Livestock Rule, enforce Pasture Rule, clarify genetic engineering or organic production. Shine a light on organic practices when asked about climate mitigation. Consider work agenda on carbon sequestration, including a restriction on ammonia extracts and highly soluble nitrogen fertilizer. Increased support for certifiers and work force in tackling fraud, including issues and work that would arise with the Strengthening Organic Enforcement Rule. NOP should provide data on un-implemented NOSB recommendations. There should be more research on impacts and resources for BIPOC farmers. Support paper-based planting aids, but there should be caution on % of non-biobased materials. BBMF is not ready for use.
Mike Dill (Coordinator for OPWC and with Organically Grown Co.)
Relies on sanitizers and experimented with various types of sanitizers. Peracetic acid and ozone performed well but workers complained about danger and odor. Workers want chlorine. Sanitizers are an outlier on the National List because OFPA does not address food safety. Sanitizer use could be addressed through more specific handling standards rather than just including on National List.
Q (Ela): We don’t have the option not to list them right now; how should we move forward?
A: Should look at the harm to human health and the environment if no sanitizers are used; people become ill or die from not having sanitizers. A do-nothing approach or the inverse would really affect humans.
Matthew Molineaux (OTCO)
Organic inspector who did IOIA crops training to become a crops inspector. Inspect about 150 farms per year. Comment on inspectors/human capital management. OTCO is hiring inspectors as full-time employees. Being an independent inspector contractor often required paying for expenses in advance and then waiting for unpredictable reimbursement. Quality of inspector work does not exist in a vacuum and the financial stress and specifics of the job need to be taken into account. More attention is needed to support inspectors.
Q (Baird): What you describe of the high levels of stress and financial instability is accurate.
Q (Powell-Palm): Thanks for the input.
Q (Huseman): What is your normal radius of travel and how does that vary?
A: Lived in Oregon, mostly worked on west coast, driving 5-6 hours a day. Often booking two inspections a day. The backend work is the report writing which takes a long time to get done. 8-9 months of the year was when most of the work was. Traveling a lot is part of the job.
Thursday, April 22, 2021: Public Comments
[Public comments were exclusively via webinar this year due to response to the COVID-19 pandemic.]
NOSB members in attendance (all NOSB members we present at the beginning of the call except for Asa Bradman, who joined later).
PUBLIC COMMENTS
Jaydee Hanson (Center for Food Safety)
Earth Day, 2021. CFS comments to National Organic Standards Board.
I am Jaydee Hanson, Policy Director for the Center for Food Safety. I will address our comments on excluded methods and BPA. My colleague, Meredith Stevenson, will address hydroponics later this afternoon.
Beginning in 2011, NOSB began dialogue and debate regarding the excluded methods definition, which resulted in a framework approved by the NOSB in the fall of 2016. This framework should be formally adopted by NOP and codified as a guidance document.
In 2016, NOSB identified eleven methods to be excluded from organic production: targeted genetic modification, gene silencing, accelerated plant breeding techniques, synthetic biology, cloned animals and offspring, plastid transformation, cisgenesis, intragenesis, agro-infiltration, transposons developed via in vitro nucleic acid techniques, and mutagenesis induced through in vitro techniques.
Without further delay, NOP should codify the prohibition in organic for these eleven methods by publishing a guidance document for the NOP handbook. All of the NOSB recommendations on excluded methods have been unanimous, which reflects the organic community’s united stance that all genetic engineering should be prohibited in organic. Delay in finalizing a guidance encourages lax and confusing enforcement of prohibitions on genetic engineering which threatens the organic label.
The unanimity of all recommendations allowing marker assisted selection, transduction, embryo rescue in plants, and embryo transfer in animals in organic reflects the organic community’s united stance. NOP should codify that these four methods are allowed in organic by publishing a guidance document for the NOP handbook to ensure clarity for all stakeholder groups
The NOSB must continue to move ahead to determine the status for the “to be determined” technologies and other GE technologies that emerge to provide clarity to all stakeholder groups. The NOSB must base such determinations on input from scientists, plant breeders, and other organic stakeholder groups and publish these determinations regularly for public comment. Failure to continue work in this area will negatively impact organic plant breeders and the organic seed industry, who need certainty to advance plant breeding efforts that meet the needs of organic operations.
We support the NOSB’s work in updating organic seed regulations and guidance in recent years, and strongly urge the NOP to adopt these changes through rule making and guidance documents. We also encourage the NOSB and NOP to continue to strengthen organic seed availability, use, and enforcement among organic growers.
- BPA and Related Chemicals
The National Organic Program must proceed with research on Bisphenol A (BPA) and related chemicals in packaging. CFS has urged NOSB to push the NOP to act on BPA and related chemicals for the last 5 years. This year, one major organic company has made a public commitment to eliminate ortho-thphalates from its products. Before companies one by one ban these chemicals from their products, NOP must implement its long delayed research into the prevalence of bisphenol A (BPA) and other chemical additives in organic packaging materials and the human health concerns associated with food contact with BPA and other chemicals used to replace BPA. Consumers choose organic products because they seek to avoid synthetic and toxic substances, and want foods that protect their health and the health of their families. Many consumers would likely be surprised that the packaged organic foods they buy to nourish their families may be in containers lined with BPA, and the continued allowance of this practice by NOP risks undermining consumer faith in the organic label.
NOP must guard against other plasticizing chemicals being used to replace BPA. Many of the substitutes are ortho-phthalates that CFS has petitioned the FDA to ban as food additives due to research showing that they are endocrine system disrupting chemicals. CFS stresses that the science indicating BPA’s capacity to leach into food when present in food packaging and the endocrine disrupting impacts to consumers make it unsuitable for packaging organic food products. CFS strongly urges NOP to prohibit other common packaging chemicals used primarily as additives to plastics and which have negative human health impacts, including ortho-phthalates and nanomaterials. The good news is that most of these chemicals do not bio-accumulate. Ban them now and they will rapidly disappear from our bodies. NOP does not need to do primary research to conclude that a ban is needed, significant research has been done to support a ban on these chemicals.
Due to human health concerns related to the contamination of foods and beverages from BPA contained in the packaging material, no organic products should be packaged in materials containing, lined with, or otherwise incorporating BPA. BPA must be fully excluded from organic products.
However, there is evidence that some organic food companies maybe replacing BPA with ortho-phthalates in plastics used in processing and packaging, and that these chemicals are similarly leaching into the food products. Research shows that high fat foods like cheese were among those likely to contain high levels of phthalates. Organic cheeses wrapped in plastic could easily have phthalates that have migrated from the wrapping to the product. We are working with the Toxic Free Food Coalition and have tested organic cheeses and the bottle caps of organic drinks. These tests show that organic products, like conventional products, will contain ortho-phthalates if the plastics contain these chemicals. As I mentioned, one significant organic company has publicly announced that it will stop using plastics that contain ortho-phthalates. We are urging the Organic Trade Association to work with its members to exclude ortho-phthalates from their products. Still, rather than pressing organic companies, one by one, to eliminate BPA and its, replacements ortho-phthalates, the NOSB should recommend to NOP that these endocrine disrupting chemicals be banned from organic products.
Otto Kramm (Organic farming operation)
Organic farming is about nurturing the soil. We questioned any input that had a readily available source of nitrogen; organic farming is a principle of working with mother nature, using proper inputs, etc. Producers are the protectors of the organic industry.
Kate Mendenhall (Executive Director, Organic Farmers Association)
Owns and operates diversified organic farm in Iowa and is executive director for OFA. Organic farming is based on principles of nature, caring for communities, thriving rural communities. Corporatization, industrialization, etc., pose severe risks to organic farming both ecological and social. We need real enforcement from NOP. The container memo opened more questions; there needs to be more clarity and does not agree with “organic hydroponic.” Clarify and implement regulations including Organic Livestock and Poultry Practices Rule, Origin of Livestock Rule, etc. If you make a mistake, fix the issue.
Q: Survey with some of the farmer members about ammonia extracts—said majority were not interested in using them, but it wasn’t unanimous.
A: Comments from farmers who wanted it were not specific on why they wanted it. We didn’t get a lot of concrete feedback. Most don’t feel soluble nitrogen is in line with organic principles.
Greg Rawlings (Jacobs Farm / Del Cabo Inc.)
[Farm located in California.] Soluble nitrogen is a problem because it breaks down the relationship between the soil and the plants. All soluble nitrogen, not just ammonia extracts. The biota in the soil provides flavor, resilience (helps with pests/disease) and moving nutrients. True organic systems need insoluble nutrients placed in soil; you need that symbiosis to be true organic. “Feed the soil, not the plants.”
Became an organic farmer for flavor, and these soluble nutrients will kill the flavor and hurt a true organic system from flourishing in your fields.
Q (Petrey): What you said about it best putting pre-plant fertilizer, can you elaborate? Do you get leaching rains; able to grow 120–day crop without issues?
A: If I have to apply after/during a cycle for an annual fertilizer than I am doing something wrong. During the winter time.
Q (Bruch): Can you comment on alternative methods you are using? Other farmers in California say they want ammonia extracts.
A: Compost that is mix of 30-40% animal manure and green waste. Best way to provide nutrients to soil long term. Lime and gypsum. Bloodmeal or feather meal for shorter crops all go in the ground. The more soluble, the more problems you will have in an organic system.
Q (Ela): How would you define highly soluble?
A: Liquid. Solid form much less likely to have issues arise. Liquid fish is problematic (you can taste it).
Harry Rice (Global Organization for EPA and DHA Omega-3s (GOED) (International trade association))
Want to increase consumption of EPA and DHA. The current fish oil annotation: GOED views modification to annotation as appropriate to address sustainability concerns. Supports “option 1” of the suggested annotations. A limitation would be the NOSB creating a list of appropriate third-party certifiers. Given this issue GOED would also accept “option 2”, which assures third-party certifiers are held to high quality. “Option 3” is not a viable option because the scope is too limited in species. The goal is that consumers have access to organic products with fish oil (because organic fish oil does not currently exist).
Dave Chapman (Co-director, Real Organic Project; Organic Farmers Association/Vermont Organic Farmers/Long Wind Farm)
I’m Dave Chapman, Co-director of the Real Organic Project. Listening to the testimony Tuesday and today, I hear a sense of loss about the National Organic Program. I don’t mean that the organic movement is defeated. But I do see many of our friends, including many of you on the NOSB, who are subtly guided to what they think is possible in the world of regulatory reform, rather than what they think is important, even crucial. We are told that hydroponics is a settled issue. Congress and the courts mostly agree.
So it is tempting for you to turn away from the big picture, to find some SMALL rule that perhaps we can reform. Or perhaps we can make better standards for soilless production getting certified as organic! Less plastic. Better fertilizers. A three year transition period. All the while ignoring that you can’t have organic farming that isn’t based in the soil, based in the fertility of a healthy soil.
In some places now, Regenerative Agriculture is seen as the defender of soil health. Organic is seen as the defender of hydroponics and CAFOs. How in the world did that happen? So as we decide where to focus our limited energies, I am reminded of that old story. One night a guy comes upon a friend on his hands and knees frantically searching under a streetlight. He asks his friend, “What are you looking for?” His friend says he lost his keys. So now they are both down on their knees searching for a very long time.
Finally the guy asks his friend, “Are you sure this is where you lost your keys?” And the friend says, “No I lost them in the bushes over there, but the light is much better here.” Let us not get seduced by the promise of searching for our lost meaning where we think we can win. The USDA is mistaken when they insist that hydroponics is a settled issue. It will never be a settled issue as long as farmers and eaters say it isn’t settled. My goal is to find a way for people to buy healthy food grown in healthy soil, the same as it always was.
In fact, I haven’t lost my keys. I know right where they are. I am just trying to help my friends who have lost theirs. And if you join us you will find quite a lot of company out there in the dark. You won’t be alone. Finally, my friend Linley Dixon asked me to apologize for a misstatement she made Tuesday. She was quoting me about the percentage of CAFO eggs in America and she misspoke. The actual (horrifying) number was that 75% of the certified organic eggs would be decertified by implementing the OLPP.
I learned this in a meeting in April 2016 that included Miles McEvoy, his boss, Elanor Starmer, head of the AMS, Sarah Eckhouse, senior advisor to Secretary Vilsack,and several others from the NOP. When I told them that failure to implement a moratorium on hydroponic certification would lead to hydro being “too big to fail,” Miles replied that if the OLPP was implemented, it would lead to the
decertification of 75% of the certified organic eggs in America. “So don’t tell me we are afraid of too big to fail.” Of course the outcome is that the OLPP was rejected, Miles, Elanor, and Sarah are gone, and with ongoing Congressional support, the certification of CAFO eggs has only increased.
Q (Jeffery): Can we really produce an equitable and diverse food system without democracy?
A: Pretty unlikely. Democracy is what we hope to see in action in the USDA and it is a struggle.
[NOTE: Cornucopia believes this question by Jeffery was meant to point out the NOSB recommended in 2017 that hydroponic/container production could be allowed, with limits. However, that reading is disingenuous to the complex history of the debate about hydroponic/container production within the organic marketplace. Nicole Dehne of NOFA-VT excellently clarified that history (see two comments below this one). For more information on the history of hydroponics within the organic industry, check out Cornucopia’s hydroponic resource page. Cornucopia also has a full report on soilless growing.]
Stephen Walker (MOSA Certified Organic)
[NOTE: this commenter provided their comments in full, and that text is reproduced here.]
Happy International Earth Day. I’m Steve Walker, from MOSA. MOSA certifies 2100 operations, and we’ve been a part of NOSB meetings for over 20 years. We have some insight on the human capital discussions and I’ll touch on some of the high points and common themes from our written comments.
Enforcement responsibilities are ever- increasing. We’re concerned thatregulatory burdens are inequitable, increase stakeholder costs, and cause burnout. We support strong standards, with detail, but compliance schemes don’t always improve integrity, and can derail organic vision. In these times in our current system, a root constraint is an inability to pass increasing financial burden on to farmers. We’re very sensitive to financial struggles many organic operators face. At the core, we need stronger agricultural policies that support living wages, and consider true cost accounting in supporting organic systems.
Capacity factors impacting inspectors and reviewers are just a p art of broad increasing demands. Beside collaboration or financial support, we need creative thinking on compliance verification methods. But, our current burdens eat the time and space needed to develop creative solutions. For example, standards could be more scale critical, as opposed to scale neutral. Operations like large dairies or importers have higher risk, but resulting enforcement mandates impact all operations, inequitably. And folks with market advantages in our current ag and regulatory system are less inclined toward systemic change. But our planet needs creative movement toward organic principles like health, ecology, fairness, and care.
To keep organic accessible, we’re seeking practical respite. Ideas include using risk assessment to focus our limited capacity, using remote technologies, revisiting the $5000 exemption threshold, and more certification cost assistance. Without relief, we anticipate more small farm certification surrenders.
Toward solutionary thinking, we support assistance for the NOSB, with more autonomy. We appreciate the breadth of skills, and time, needed for effective policy work. Non-prescriptive assistance with your busyness and burden can better enable your necessary creative and critical thinking. NOSB members should decide where or how you need support. However additional support is used, we expect the NOSB to author your own proposals and documents.
Related, as private representatives in the public-private partnership, we support more work plan autonomy, with balance. For some festering enforcement concerns, NOSB discussion could have great value, but these are not finding their way to work plans. Conversely, some past agenda items seemed an inefficient use of your time.
Q (Baird): Can you elaborate on your risk assessment ideas? IFOAM has a risk assessment for their group certifications; should re-visit.
A: If we have to hold every operation to the same measures, that does not always make sense. We need more time and capacity to dig deeper on other operations; don’t need to hold every operation to the same check-list, just the same standards. The ACA had a working group awhile back to determine risk assessment criteria. Effort is to come up with quantitative criteria to determine qualitative factors.
Nicole Dehne (Director for NOFAVT)
[NOTE: this presenter provided their comments in full, and that text is reproduced here.]
My name is Nicole Dehne, I’m the director for Vermont Organic Farmers representing over 800 organic producers in Vermont. I would like to thank the NOSB for all of your hard work and for the opportunity to give comment today on a number of agenda items.
1) Hydroponics & Container Production
The issue of certifying hydroponic production as organic is not resolved. We disagree with the assertion that hydroponic operations have been allowed to be certified since the NOP began. A review of past NOSB meeting transcripts clearly shows this to be untrue.
According to the NOSB transcripts from April 1995, the board’s recommendation was to consider allowing hydroponics to be certified if “very strict conditions that -the board would elaborate in guidance material” were met. This discussion of guidance and needing clarity around whether and how hydroponic operations could be certified continued until the 2010 NOSB Recommendation.
The 2010 Recommendation clearly prohibits hydroponic production, provides past discussion documents on this issue, provides needed definitions, and creates a new section of the crop standards which defines how plants in containers and enclosures can be certified.
It is unclear why the NOP has not taken up the 2010 NOSB recommendation prohibiting hydroponics and adding the suggested parameters for container production to the crop section of the NOP rule.
As an industry, we still need guidance that allows consistency, that provides transparency for consumers and ensures a level playing field for producers. We strongly recommend that the NOSB add this issue to their work agenda and collaborate with the NOP to determine what clarity is still needed in order to finalize the 2010 NOSB Recommendation.
2) Proposal: Biodegradable biobased mulch film annotation change.
We are grateful for the work that board has put into this complicated topic of reviewing BBM and we support the proposal.
We agree that we want to be careful about the effect of synthetic polymers and their potential to accumulate small particles of plastic in the soil. Research on this topic should continue. However, this research should be extended to include other areas of risk for micro plastic contamination- that includes the use of polyethylene mulch as well as the use of compost where food waste is a feedstock.
We would suggest that the current listing be amended to remove the phrase “Must be produced without organisms or feedstock derived from excluded methods.”
Doing this would ensure that BBM is evaluated in the same way as other materials on the National List. It would still prohibit any products that rely on genetic modification and instead would only allow materials with “incidental” GE usage as long as the GE modification is not expressed in the final product.
Q (Caldwell): How would hydroponics/container systems relate to our strong focus on organic promoting biodiversity and carbon sequestration?
A: It does a disservice to our messaging in the industry right now. Allowing soil-less production hurts that messaging.
Q (Wood): Appreciate broader risk-assessment for microplastics. Are you concerned about the use of compost derived from food waste?
A: Right now, in Vermont, all the food waste with the plastic packaging is chopped up together and then the plastic is separated—but about 1-3% of the compost is plastic pieces. Microplastics are then in the compost and being applied to organic farm fields.
Q (Ela): Can you clarify about your recommendation to remove or modify phrase about excluded methods in mulch?
A: When we add the annotation, BBM would have a higher standard than other materials on the National List when considering excluded methods. Because sometimes it’s hard to determine if something is genetically modified or not. Sometimes bacteria is being fed by GE corn, for example, but the GE corn is not in the final product. Leaving this annotation in means that would not be okay for BBM to have that connection to excluded methods, but it would still be allowed for other substances (like compost, organisms and dairy cultures for example), on the national list.
Garth Kahl (Common Treasury Farm, Independent Organic Services, Inc./organic inspector)
Supports proposal for support staff for NOSB members. Do not remove ion exchange from organic (organic stevia). Human capital: He is an IOIA trainer has the capacity to train inspectors, but it does not address broken apprenticeship program. We need better inspection process; IOIA has created pilot program which needs to be supported by organic industry. Credentialling makes no difference in what certifiers are willing to pay at this point. We need to support a professional class of inspectors with specialization.
Q (Ela): The recharge materials need to be on the National List; but should resins appear on National List?
A: Question of food contact surface; if resins are a food contact surface, we need to look at other surfaces like the insides of barrels, sealant rings, etc. If you are going to look at resins or possibly require listing them, please make it not disruptive to the industry.
Q (Petrey): I agree on the specialization of inspection being important (with commodities); do you think specialization is more important than location?
A: We need training that increases capacity of inspectors in all regions. We need both localized and specialization. We need to look at risk-assessment as well; we need specialists and local strength. It’s not sustainable to fly inspectors all around the country and all around the world.
Q (Powell-Palm): Do you only find fraud in larger operations? How mature is the program that IOIA is putting on?
A: No. Failure to meet 30% DMI, for example, is found in all scales. Fraud is happening at all scales. Larger entities have a brand to protect moreso. The IOIA pilot program has proposed budget and brands can make a contribution on the IOIA website.
Q (Kim): How long is the apprenticeship? What’s the amount of time to decrease learning curve for inspector?
A: Five days on the ground with a mentor inspector. Write five inspector reports. Followed by one day of didactic management of various kinds. Another component: ongoing mentorship for one year after apprenticeship.
Francis Thicke (General public)
Speaking on behalf of 40 former NOSB members who served on the Board from 1992 to the present, Thicke shared excerpts from a letter to USDA Secretary Vilsack, highlighting their concerns about the erosion of the organic standards.
[Cornucopia Directors, Goldie Caughlan and Kevin Engelbert, and three of our advisors, Dr. Joan Dye Gussow, Dr. Fred Kirschenmann, and Dr. Jennifer Taylor, are among the signers of the letter.]
Jennie Landry (DSM Nutritional Products)
Fish oil annotations comment; DSM supports annotation options #1 and #2. Support sustainable and renewable environment. These annotations are clear and enforceable. It is critical to allow as much flexibility as possible; it’s important for diversified supply chain. Option #3 is not as clear or enforceable. Fish oil manufacturing supply chain can be complicated; not feasible for a downstream manufacturer to verify suppliers.
Aaron Gardner (Senior Specialist for Handling, Organic Valley/CROPP Cooperative)
Comments on animal enzymes and cellulose. Animal enzymes are used in production of blue cheese/aged cheeses. These cheeses do not hold up with alternative vegan enzymes. We support the continued listing. Cellulose is used in two applications: casings for organic hot dogs and sausages and shredded cheese. They are essential ingredients and the only alternative ingredient are “plastic cases” and they do not function in the same way. Powdered cellulose is used for shredded cheese and grated Parmesan cheese.
Q (Huseman): Animal enzymes and the availability of enzymes from organic livestock—are there opportunities or barriers?
A: There is not the number of organic livestock available to provide us with the amount of the enzyme that would be needed for production.
Harriet Behar (Organic farmer and advocate, Sweet Springs Farm)
Former NOSB member. Ammonia extracts: NOSB needs to review all highly soluble fertilizers and develop a use policy. Recommend use limited to “emergency use” for when events occur that are not normal for that region. Allowing highly soluble fertilizers at any time mimics conventional agriculture. Organic farming does not just substitute inputs, but instead builds soil resilience as the farm matures.
Ion exchange materials: do not give the NOP more say over the materials list.
The NOSB should have control over their own agenda. The review of greenhouse/container production was approved as work agenda item and then removed by the NOP; this is problematic. Please push the NOP to implement Native Ecosystems. Refer to NOC comments on paper pots and BBMF for Behar’s thoughts.
A: Interesting to restrict use to “when needed”; would you expect tissue analysis like calcium chloride?
Q: Similar to other items in pest management hierarchy; show that you tried to respond with other methods but there were climatic conditions that required you to use highly soluble fertilizers. It would indicate a problem with the system if you had to use year after year. Should only be used when the system has failed because of outside pressures (like weather).
A: How would you define highly soluble fertilizers?
Q: “You know it when you see it?” Liquid feeding given to plants that would have a quick uptake.
Meagan Collins (Accredited Certifiers Association, Inc.)
Human capital management: the proposal does a good job outlining the problems. Organic certification process is complicated and globalized. Greater need for more qualified inspectors and ongoing training. Certifiers would need to increase the cost to farmers if they increased pay to inspectors. We do not know what certifiers pay their inspectors or whether larger inspectors are paying more to out-compete, etc. We need more information and standardization concerning inspector pay. Tools should be utilized to allow more flexible work-life balance for inspectors.
A (Caldwell): What about conflict of interest with organic farms paying for their own inspector and engaging in these relationships?
Q: If we value organics, Congress should step up and consider mitigating costs and conflicts.
Tim Stemwedel (Owner of California Organic Fertilizers, Inc. and agronomist/consultant)
If natural ammonia fertilizers are placed on prohibited substance list, it would be next to things like arsenic, but it’s an essential nutrient. The petition is full of inaccurate information that refers to synthetic ammonia. The natural ammonia is the same as other natural inputs, including stimulating soil biome. The TR repeatedly states that there is no information on natural ammonia and instead speaks to synthetic ammonia. A new TR should be done by soil scientists. Farm plans require diverse use of nutrients; liquid nitrogen products are a tool to be used “as needed” and will not be the sole source of fertility.
Q (Dimitri): Can you clarify the social justice issues of other inputs?
A: Seabird guano, for example, is being harvested by poorly paid workers. Liquid fish is coming out of areas that are severely damaged environmentally and work conditions are akin to slavery.
Q (Bruch): [Quoting from commenters written comments] Concern of most fertility for strawberries in CA coming from extracts and manure.
A: Drip irrigation is common in CA with some crops because of long in-ground time. Liquid products are important for that. Crop nitrogen demand is a curve that is not synchronized, that’s why we need multiple inputs.
Daren Stemwedel (California Organic Fertilizers, Inc.)
Comment on natural ammonia; oversees manufacture of products and is also attorney. Advocates for preventing fraud in organic fertilizer production. Singling out one fertilizer for a fraud risk is unfair because all fertilizers are a fraud risk. Natural ammonia products are less susceptible to fraud because there is more scrutiny of them. Input fraud is very rare; due to food safety rules there is a high degree of traceability with organic fertilizers.
The TR should be discarded; no effort was made to obtain data on natural ammonia.
Rhoda Benson (Technical Issue Specialist Northwest Horticultural Council)
Represents apple, cherry, and pear growers in the Northwest. The Council supports the multiple listings of sanitizers and disinfectants; see their written comments. Critical for growers and packers to clean and sanitize their food to protect the public health. We need multiple products, not just one; growers and packers have to combat many types of pathogens.
Sarah Pinkham (OEFFA Certification)
Crops Subcommittee: Due to issues of consumer trust and organic integrity, we cannot add kasugamycin. BBMF is desirable but the proposed annotation allows products made from petroleum which should not be allowed. Continuous improvement means saying no to these inputs.
Lack of consistency undermines consumer trust. NOSB must maintain its responsibilities under OFPA and continue to recommend regulatory change. Do not pass costs onto the smallest farmers; see written response to human capital management.
Maria Ignosh (Shenandoah Growers, Inc.)
If ammonia extracts are allowed, an annotation should be added. Produce fresh premium organic herbs; working on their bioponic/bio-filtration method of supplying nutrition. Use a nutrition program that uses ammonia extract, using organisms to convert the ammonia to a nitrate solution that is then fed to the crop. The ammonia extracts are important. We perfected this method to be in compliance with organic standards. Ask that an annotation would allow for using it as a nitrate feedstock for these kinds of growing systems.
Q: How do your production methods jive with requirements for biodiversity and carbon soil sequestration (which is not a requirement, but being touted)?
A: We do both indoor and greenhouse production. This is a very efficient growth method because we are stacking plants; not much land used. A lot of our facilities are built in existing facilities. Efficient water and nutrient use so not releasing nutrients into the environment. We grow where we need the product to be which is an efficiency advantage.
Q (Ela): If we were going to prohibit them, we should allow them if they would be bio-converted into nitrate. Doesn’t that conversion occur in the soil as well, so that would be pointless?
A: My colleagues should address this; I do think that’s what happens in the soil.
Bill Denevan (Viva Tierra Organic and the California Apple Commission)
Fire blight will destroy a grower more than any other pathogen or pest. Kasugamycin can help stop fire blight. His experience is that it is financially devastating when fire blight goes through an orchard. It is especially vulnerable for farmers in California, but cooler areas are at risk too. With climate/weather changes, more alternating materials are needed to present resistance.
Q (Caldwell): Are there combinations of scions and root stocks that show better performance than others?
A: No difference; climate is the biggest factor (not having winter chill is risk factor).
Q (Bruch): Fire blight is more prevalent in California and the Pacific Northwest; is there a soil health component? Are their soil deficiencies in these areas?
A: Can be related to too much nitrogen. Young trees with too much nutrition can die faster than an older tree with less nutrition. You can’t go over the top with sprays or nutrition.
Q (Ela) How do you reconcile the NOSB voting against the use of any antibiotics in crops and then suddenly adding kasugamycin?
A: Kasugamycin is a different kind of product because it’s not used in human or animal applications.
Nate Lewis (Oyster Bay Farm)
Farmer in Olympia, WA. Organic crops, beef, lamb, chicken, eggs. Ammonia extracts: encourage the NOSB to move forward with full prohibition; they are not necessary and not compatible with organic production. We already have high nitrogen fertilizers that release more slowly. Someone should take a leadership role and develop an action plan to ingrain equity into everything the USDA does, using the NOSB as an example.
Q (Ela): In terms of diversity issues, what can the NOSB do to move things forward?
A: Washington Farmland Trust is in an area fraught with equity issues; NOP should provide a budget to hire an outside consultant to guide NOP and NOSB through that process.
Jackie DeMinter (Certification Policy Manager, MOSA Certified Organic)
Summarizes written comments on BBMF, sunset materials, and paper pots. Support the use of paper as a production aid, ask that the NOSB pass the proposal. Should move swiftly to approve. Almost 500 operations certified by MOSA use a mulch (183 farmers use a plastic mulch), and BBMF would be desired by the market as an alternative; support the 80% annotation with some caveats on the annotation language and details. Should review all current mulch film products to see if there are any that meet the standards. Livestock sunset: electrolytes are commonly used; injectable vitamins are widely used but no MOSA clients are currently using zinc sulfate for hoof care.
Q (Bradman): Can you speak to your concerns about the use of the word “plastic” with the mulch films? Clients raised concerns about petroleum content in films and paper pots?
A: Just for consistency in the use of the terms within the regulations; issue of consistent language. Have not heard any concerns coming from clients or staff about those issues.
Q: Are MOSA producers using copper sulfate instead for livestock?
A: See our written comments. Almost 100 clients using copper sulfate.
Q (Ela): Re: the word plastic, we were worried about paper mulches already out there and that growers would be forced to use the paper mulches.
A: The paper would fall under the paper listing, while BBMF would fall under this listing. Would need to give that more thought with a proposed rule.
Peggy Miars (OMRI)
Skipped/not present
Todd Linsky (General public)
TLC organic consulting. Comment on the certification of next generation farms. Where we are headed is concerning because we are disassociating from the next generation of farmers by excluding new practices. The next generation of organic farms are no longer in the dirt; they can grow almost anywhere and have a platform and a story to share.
The move to exclude non-traditional growers is a problem, especially because of food insecurity. Soil is one element of the ecosystem and the bigger picture view looks at everything that supports life.
Q (Ela): One of the things we are governed by is OFPA. How would we reconcile with what you are saying with the regulations?
A: Need to talk this thing through all together, greenhouse and soil–based producers. This technology is not going to go away. Hurts organic brand by not coming together.
Emily Musgrave (Organic regulatory manager, Driscoll’s Inc.)
There is a need for a bio-based alternative to plastic mulch. The subcommittee should differentiate between two manufacturing techniques for ammonia extracts. Support annotation for 80% bio-based BBMF if there are manufacturers that can meet that requirement. BBMF is a needed tool; plastic mulch is going straight to landfill.
More research is needed on effects in soil, but this research is in process. The longer we wait, millions of pounds of plastic mulch are going to landfills.
Ammonia extracts petition is too broad and does not differentiate between the two different manufacturing processes involved. Requests NOSB create a working group.
Q (Bruch): One of the proposed annotations for BBMF is for 90% biodegradation in two years. Given potentially significant annual usage of this product, will it complicate the determination of how much breakdown occurs in two years?
A: Agronomy team is working with Washington State. I will send this question to them.
Q (Ela): In terms of the two processes for ammonia extracts you noted, would you allow one and not the other?
A: From what I know, ammonia concentrating should be allowed, not ammonia stripping. Ask Allen Philo.
Gina Colfer (Wilbur Ellis Co.)
Supports use of ammonia extracts; commenting on behalf of Wilbur Ellis. Pelleted and liquid organic fertilizers are commonly used in CA because cover crops are not predictable in this climate. Ammonia extracts contain no phosphorus, unlike the currently available nitrogen fertilizers. Ammonia extracts are designed to be spoon-fed (rates of 10 gallons per acre). They are not comparable to sodium nitrate in any way. Thorough and unannounced inspections should be part of the process.
Q (Caldwell): Why would sodium nitrate products be unacceptable while ammonia extracts are?
A: Sodium nitrates are mined in Peru and give a sodium boost at the same time as they deliver nitrates.
Q: What type of ammonia is in this product?
A: 8% nitrogen, half ammonia, half nitrates.
Q (Bradman): Is pelleted chicken manure coming from organic or conventional sources? What are the liquid fertilizer sources?
A: Noted that pelletized chicken litter is sourced from farms around the country with additions of blood meal, etc. Based on the growing stage of plants, they may be unable to take up all nitrogen, allowing leaching from the fields. Liquid fertilizers are based on fish emulsion, soy hydrolysate, etc.
Q (Bradman): So, the chicken pellets rely on conventional ammonia upstream. (Conventional chickens eat feed produced using conventional ammonia sources.)
A: Yes.
Q (Jeffery): (Not available)
A: Not an inexpensive process to make ammonia extracts; the products will be $11-12 per unit of nitrogen. Fraud could be discovered if the product is cheaper than that. Growers should use due diligence and note the improbably low cost.
Peter Johnson (Pete’s Greens)
Skipped/Not present
Kestrel Burcham (Policy Director, The Cornucopia Institute)
See Burcham’s full comments here.
Jim Koan (Owner/operator of a certified organic fruit farm in Michigan)
Grew apples conventionally for 20 years, has now grown organically for 24. Has disrupted coddling moth with pheromones. Sell apples for $1 per pound, and takes pride in making affordable, organic food for people. If pheromones were not allowed, apples would suffer 30-40% damage, and he would have to double his price. There are no other tools that are as sustainable. Kasugamycin would be a useful tool.
Q (Greenwood): Interested in kasugamycin comments. There were hundreds of comments about keeping antibiotics out of the food chain. How would your customers feel about antibiotic use?
A: You make a decision based on sound science. If you find there is harm to the environment, okay. But we ought to educate consumers if not.
Comment (Greenwood): Good evidence that it changes the soil microflora, but I’m not sure to what extent.
Q (Ela): Can you clarify what you’re currently doing to prevent fire blight?
Transitioned to more fire blight resistant varieties. Uses a lot of copper close to bloom, minimize nitrogen application. Still lose trees. Kasugamycin would save trees.
Ryan Brandt (NuOrganics)
Petition for ammonia extracts: strongly oppose the petition; the definition is too broad and needs to be refined. They process fertilizers from manure. Manure has some downsides, such as too much phosphorus, that their ammonia fertilizer, which is OMRI approved, can overcome. Using raw manure to create a dry crumble fertilizer and a liquid fertilizer allows farmers to apply nutrients in optimized amounts. Natural ammonia fertilizer has soluble organic carbon in it, which fingerprints the natural products and feeds the soil biome. Manure-derived products are uniquely transportable, allowing organic farmers access to these products where they didn’t have access in the past.
Q (Ela): Ammonia extracts unlock other nutrients in the soil where ammonia extracts in conventional ag do not—can you clarify why this is?
A: We separate the raw manure from the solid fertilizer; allows you to then prescribe the precise application of nutrients to the crop.
Allen Philo (BioStar Renewables)
Comment on ammonia extracts. Read the white paper written by Dr. Jerry Hatfield and Philo about ammonia extracts. Ammonia fertilizers in field trials show that total nitrogen released is higher than the applied ammonia, feeding the soil and not the crop. There are long-standing assumptions about how fertilizers operate in the organic community; the NOSB should slow this process down and form a task force so that all the scientific and technical data can be directly found and addressed.
OMRI did not err in approving natural ammonia fertilizers.
Q (Amy): Big picture question: when performing these tests what kind of soils were you working on?
A: Base conditions were around 4% very good soils in WI area, well balanced, 7 pH. The soils had applications of other fertilizers like chicken manure. When we applied 6 pounds of nitrogen, we ended up with significantly more yield, showing it stimulated biology to break down more of the other added solid fertilizers and make that available to the plant. Will send actual study.
Q (Ela): Were soils certified organic? Long term studies? What would you apply year after year?
A: No, soils were not organic but it’s impossible to find folks to do organic trials. Organic systems work on having active good biology in them so having conventional soils is a downside. Only one year of research but we have repeated it over six different crop types and have seen the same pattern. The base nutrients we are speaking to are the other fertilizers, like chicken manure, that were applied. Typically, you only credit 30% of the chicken manure to nitrogen, but adding this product credited over 50%…
Chris Schreiner (Executive Director, Oregon Tilth)
Human capital: As a certifier, we redesigned our inspector team to full-time staff instead of contractors. Building the pool of qualified inspectors requires a retention strategy. We’ve persuaded them by responding to quality of life, career stability, insurance coverage, competitive salaries, etc. This stabilizes the pool of organic inspectors. Keep the best we have and incentivize the rest. Funding should be provided for mentorship matching and services. As organic certification become smore complex, costs are increasing; the pandemic is an opportunity to evaluate new technologies like remote inspections. Risk-assessment for inspection priority is needed.
Q (Powell-Palm): Your feelings on creating a more standardized mentorship program, like administered by IOIA?
A: Consistency is key. A standardized system will help increase that consistency.
Q (Caldwell): Did hiring them increase your costs and did you pass that onto farms?
A: It’s brand-new; shifted costs from contractors to payroll and benefits. There are some increased costs. We have standardized an hourly rate for inspection services, so clients will not see as much variation year-to-year. Expecting a more consistent price experience.
Q (Smith): Working with universities/land grants; challenging to get organic courses in there to speak to organic as career path. What would that outreach look like?
Q: Oregon Tilth has a partnership With Oregon State University, a strong land grant university. Advice: engage with universities, bring credible science and engage administrators and faculty. Tilth has invested in OSU’s programs; our financial commitment grants some access to setting university agenda going forward.
Michelle Miller (General public)
Tech Entrepeneur that was poisoned in mosquito abatement. Please don’t compromise organic food standards. Be creative and solve problems in other ways rather than compromising the standards. Do not allow kasugamycin, please don’t change annotation for BBMF, and please label ion exchange materials.
Michael Hansen (Senior Scientist with Consumer Reports)
Carrageenan: we oppose its re-listing. Supported fall 2016 vote to remove carrageenan due to its lack of essentiality and potential adverse health effects.
Kasugamycin: we oppose listing. Organic should mean no use of antibiotics or other drugs. It’s not permitted in other countries and there is potential for antibiotic resistance. The Technical Review states that the normal use of kasugamycin has already led to resistance in some pathogens.
Excluded methods: move forward with NOSB recommendations and urge NOSB to note other methods up for review. Specific definitions should be developed on what is and what is not excluded.
Q (Caldwell): Should cell or protoplast fusion be approved?
A: Modern biotechnology definition would ban cell fusion, but only above the level of the family. I don’t think it should be used at all.
Q (Jeffery): Does CR have current survey data about excluded methods in organic?
A: When we’ve asked, anything that is considered GE should be excluded along with pesticides and drugs.
Q (D’Amore): Have you looked at environmental concerns for carrageenan?
A: Other groups like NOC have referenced adverse environmental effects. The lack of essentiality, human health, and the fact that other programs do not allow it—that is enough to ban it.
Ramani Narayan (Michigan State University)
Plastic mulches are fragmented into microplastics, which is documented to have bad effects on soils. BBMF should be permissible even with today’s regulation, provided it is recovered for on-farm composting. Bio-based content issue is holding this back. The biodegradable requirement is that there is no bio-carbon remaining; minimum bio-based content would be useful but to wait for 100% content is holding back this valuable tech which would prevent accumulation in the soil.
Q (Bradman): We are proposing 20% non-biobased but still biodegradable. If climate is such that even biodegradable mulches break down more slowly and we can find microplastics that are persistent, that’s a concern.
A: The bio-based film is needed and useful, it’s better than what we have now, since plastic mulch films are currently allowed. Bio-based carbon has nothing to do with whether it biodegrades. If you wait for 100% bio–based it is unnecessarily delaying.
Q (Bruch): Biodegradation and plowing. Plowing is 6+ inches, concerned about level of microbial activity still active at that level.
A: There is not perfect data on what happens. We have anecdotal data. If you permit it to be removed and do on-farm composting, then the fragments that will go into the soil will not accumulate/or be persistent because we know it biodegrades completely. It will improve what exists today with plastic mulch. We don’t know the timeframe because soils are different. In a lab scale, within two years no carbon remains from these biodegradable films. It gets more complicated from particularities in the real world.
Q (Ela): Do products exist that would meet this annotation?
A: Yes, except for bio-based content at 100%. If you did not require 100% bio-based then there are products from multiple suppliers that can meet the biodegradable requirement. The part holding it back is bio-based content. The 80% level would be hard to meet as well.
Denae Ranucci (Quality Assurance International)
Audit team leader. Issue of human capital: there is a lack of qualified inspectors and it will only get worse with the implementation of the Strengthening Organic Enforcement Rule (SOE). Completely behind the curve on increased strain in inspector groups. The increased qualification requirements will be further strain on the inspectors. Increased inspections from the SOE with dwindling resources is going to create more issues that inspectors are leaving over. Encourage NOP to continue hybrid inspections we are doing in pandemic, as it will reduce travel burden. Professionalism is low in the industry; they don’t get much respect compared to other types of inspectors. There should be additional guidance on how to address inspection refusals. Additional education to the public and clients will also help.
Barbara Wingler (NuOrganics LLC)
Environmental engineer. Addressing fraud in liquid fertilizer; spectroscopy can be used to detect fraud. It can be used on dry and processed manure and are now developing the process to use on liquids. Liquid fertilizers have carbon carbons that link to the plant/animal they come from. Infared spectroscopy requires minimal training, is fast, inexpensive, reproducible, precise, and widely used in the food industry. It allows a fingerprint of carbon compounds present.
Sam Welsch (OneCert, Inc.)
[NOTE: this presenter provided their comments in full, and that text is reproduced here.]
The final rule was published with an effective date of April 21, 2001. That was exactly 20 years ago yesterday.
The preamble to the rule includes the statement, “During the 18-month implementation period, the NOP intends to publish for comment certification standards for apiculture, mushrooms, greenhouses and aquatic animals.”
The absence of those missing standards has resulted in huge inconsistencies among certifiers. The certification of soil-less systems is one of the most egregious. Such systems can only be certified by designating all the soil-related provisions in OFPA and the regulations as not applicable.
Designating soil provisions as not applicable to soil-less systems resulted in the three-year transition requirement also being considered not applicable by some certifiers. That was a logical result because the soil on that land was not part of the production system.
In response, the NOP issued a memo titled, “Certification of Organic Crop Container Systems” in June 2019 clarifying that the three-year transition period applies to all container systems built and maintained on land. That memo created more confusion and inconsistency among certifiers. The ACA formed a working group to address the inconsistent interpretation of the memo. The working group concluded that the memo only clarified that it is prohibited to spray synthetic herbicide to the land, apply a layer of plastic, put containers on the plastic, and certify the production system as organic.
The working group also identified many areas of inconsistency for certification of greenhouse and container production. Clear and specific standards are needed. The group wrote, “This needed clarity and specificity cannot adequately be achieved through certifier consensus or additional NOP guidance.” We need the NOP to draft regulations based on the NOSB recommendations on greenhouse and container production from 20 and 10 years ago, respectively. The group wrote, “These areas of inconsistency and related concerns need to be addressed through the formal public rule-making process and the publication of greenhouse and container production standards.”
I want to comment briefly that Ion Exchange is a method that creates chemical change. That is why it is called ion exchange.
Regarding so-called “biodegradable” plastic mulch, remember that OFPA clearly states, “For a farm to be certified under this chapter, producers on such farm shall not—use plastic mulches, unless such mulches are removed at the end of each growing or harvest season.”
Finally, please review my written comments on “Clear Legal Writing and the Rules of Textual Interpretation.”
Ib Hagsten (Organic inspector)
Human capital management: Not well paid as an organic inspector, but expected to be treated well by certifiers. Encourage NOSB, NOP, and IOIA to develop a recognized inspector credential program to give recognition to inspectors who raise the bar. Formalize mentorship for new inspectors; mentored new inspectors himself, without payment. There are not enough mentoring inspectors. The Organic Livestock and Poultry Production Rule not being passed is disappointing; disappointing to see deviation from the organic standards by some farms (in dairy and poultry). IOIA can’t do it all alone.
Yesenia Fuentes (BioSafe Systems, LLC)
Manufactures biodegradable disease control solutions. Peracetic acid (PA) is valuable as a sanitizer in multiple arenas. Strongly support its continued use and listing on National List. It’s effective when applied as a stand-alone product.
Margaret Scoles (Executive Director, International Organic Inspectors Association)
Human capital proposal: written comments included an inspector survey. Leading worldwide training and networking organization for organic. Too few are becoming inspectors even after training because they couldn’t get work due to not having mentorship. Apprenticeship has been a barrier as mentors should be compensated. Pilot programs with universities should be encouraged. Diversity, equity, and inclusion is essential.
Q (Baird): Can you update on the pilot program?
A: In early stages of mentorship pilot program. The funding is proving tricky; Organic Valley was the first donor for the program. We hope that participants won’t pay anything; people will apply and we will pick the most promising candidates.
Melody Morrell (Executive Director, The Cornucopia Institute)
See Morrell’s full comments here.
Kelly Taveras (Digital Specialist for Organic Trade Association)
Virtual platform for comments and NOSB meeting is important for accessibility. [Explains OTA membership and role in the industry.] OTA created an electronic survey for each individual input under review (written comments include survey results).
Johanna Mirenda (Organic Trade Association)
Farm policy director. Support continued allowance of paper planting aids; there are some technical clarifications needed but they are non-substantive. BBMF: Unclear whether regulatory solution presented at this meeting will meet goals. Support continued research and regulatory opportunities to reduce reliance on plastic mulch. Ammonia extracts: more resources and information are needed to make a sound decision to differentiate these products and make a decision. Compatibility with organic and other requirements for analysis can be subjective.
PPM: refer to NOSB recommendations form 2001 & 2004 with compatibility of substances with organic principles and consistency with organic farming. These are predecessors who gave some guidance on how to approach decision-making.
Q (Bruch): Do you have concerns about ammonia products and exports?
A: Concerns are speculative with sodium nitrate, which has proven to be a trade barrier. Not sure if ammonia extracts will have the same consideration.
Gwendolyn Wyard (Organic Trade Association)
Vice President of Regulatory, OTA. Excluded methods terminology: support the recommendations, the tech that are listed in the chart are consistent with current regulation. Many NOSB recommendations have not been acted on, including excluded methods. It may be necessary to stop working on the excluded methods chart so that regulation can be passed. Ion exchange filtration should remain, along with recharge materials, on the National List. Materials and resins should be documented and approved in each plan.
Zen Honeycut (Director, Moms Across America)
We agree with all of the statements made by Beyond Pesticides. There is a connection between the heavy metals and glyphosate as a chelator pulling metals into food. Fracking water and CAFO manure should be banned from organic production; organic standards should phase in regenerative practices. Moms switching their children to all-organic diets saw health and behavior improvements in their children. Exercise the precautionary principle.
Evan Axelbaum (Front Axle Farm, LLC, also member of OEFFA)
Consumers are more confused than ever. More small producers are forgoing certification in part because they feel organic means less and less. Stakeholders need to work on rules for container systems. NOSB needs to have control over their work agenda so that they can work on the container issue again.
Veronica Borne (CP Kelco)
Supports maintaining carrageenan on the National List. Has long-standing use as a food. Carrageenan is widely used in the food industry and gives organic operators more flexible. Carrageenan can form gels in the presence of cations, which is very useful in plant-based products.
Q: Can you use just Irish moss instead of extracting carrageenan?
A: You might have a very strong seaweed taste, which might be alarming to consumers. I can inquire further if this is possible.
Q: Some suggestions from comments that some types of carrageenan are more harmful than others?
A: I have no knowledge of that. Will get clarification.
Jessica Knutzon (CP Kelco)
Carrageenan: CP Kelco collaborates with seaweed farmers in Zanzibar. Farming provides employment opportunities and funds communities. The science surrounding harm from carrageenan is bad; carrageenan is not poligeenan. Should not remove an additive only due to consumer perception and not facts.
Jennifer Taylor, PhD (IFOAM North America, Co-president)
[NOTE: These comments were provided in full to the Cornucopia staff, and are reproduced here.]
Thank you, Administrator, NOSB members, and staff. I am Jennifer Taylor, former National Organic Standards Board member, speaking today in my role as CO-President of IFOAM North America.
IFOAM North America is a regional body of the IFOAM-Organics International. IFOAM has members in over 100 countries and territories.
Our work builds capacity to facilitate the transition of farmers to organic agriculture, and advocates for a-policy environment conducive to agroecological farming practices/organic farming systems and sustainable development while addressing the broader mission of Organic 3.0.
The goal –of Organic 3.0 is to enable a widespread uptake of truly sustainable farming systems and markets based on organic principles– that facilitate a culture of innovation, of progressive improvement towards best practices, diverse ways to ensure transparent integrity, of holistic systems, inclusive collaborations, and of true value pricings.
A number of North American organizations are working towards similar goals.
And as helpful as it is, the National Organic Program only touches on a few of these indicators in the scope of its work.
We in North America are experiencing an opportunity to build back better NOW. Let us work together to promote social justice, inclusion, participatory community engagements participatory capacity building, let us work together to support Black and indigenous farmers and farmers of color and their communities and underserved small farm populations and their communities -in their ACTIVE equal participation in building back better with inclusive networks of organic farming systems, and organic agroecology farms, participatory education, participatory hands-on trainings, and technical assistance that support resilient healthy organic food systems, local, black and indigenous community food ways, access to land, access to viable alternative markets, and safe working conditions.
Let us work together to proactively support organic farmers, and underserved small farm populations and their communities, and the principles/benefits of organic agriculture building healthy soils, healthy environments, healthy foods, and healthy communities; and enabling thriving organic livelihoods and wellbeing–for all.
Join us as we build back better, a more inclusive, socially just, equitable and sustainable participatory -organic agriculture that benefits all communities and all environments.
Sandy Mays (Partner with Wolf & Associates)
Inspector qualifications vary widely. The quality and integrity of the inspection is dependent on the inspectors. Staff inspectors may address the problem of professional inspectors. However, this approach cannot be universally adopted. Certification costs are already out of reach for many operators, so inspector costs cannot be passed onto producers. Certifiers also interpret the rules and pass this interpretation onto inspectors, which leads to more confusion. Should explore established professional standards.
Q (Smith): Are you expecting standards to be overseen by the NOP?
A: Absolutely.
Lee Frankel (Executive Dir. For Coalition for sustainable organics)
Support policy to continue to certify container systems. [Summarizes recent court case finding in favor of USDA.]
Q: The organic community has promoted increasing biodiversity, soil health, and now sequestering soil carbon. How do hydroponic operations help with that? Are there life-cycle analyses including production of plastic and resources for greenhouse production?
A: Each operation will have different site-specific conditions; woodlands and grasslands that we leave untouched sequester even more carbon than organic farms. Compost is still used/made into organic teas, so there is still cycling of resources in container production. Have not seen life-cycle use study for organic. Biggest energy use is that some producers may be cooling their greenhouses and facilities, but often that is counterbalanced by having the produce must closer to the market. Haven’t seen a good study.
Bryce Lundberg (Lundberg Family Farms)
Request renewal of copper sulfate for rice production. Never seen an impact to wildlife; applied in small amounts and is only active for a short time. Tried drill seeding but weed pressure was too high; water flooded was best. Grass weeds are managed by drowning. If shrimp appear before a seedling is green, they can destroy a crop. Algae and shrimp can be controlled by copper sulfate.
Bill Wolf (Wolf & Associates, Inc./Second Star Farm)
50th year working on improving organic. See written comments. The NOSB should receive more support organizing, collating, and reporting on comments, as well as help with drafting recommended language so it’s ready for the NOP. Think like an earthworm.
DeEtta Bilek (OFARM)
The purpose of OFARM: Establish and maintain sustainable prices for organic farm production through coordinated efforts of organic farmer cooperative marketing groups while protecting and defending the organic standards and promoting environmentally friendly production practices.
Fraud in organic grain area: once the Strengthening Organic Enforcement Rule is in place, members think it will address much of the concern with fraud. Concern with ammonia extracts due to problems with leaching and fraud.
Angela Schriver (Schriver Organics)
Row-crop farmer. USDA organic and cider e-mail said “continuous improvement is a priority” NOSB should habitually weight all requests and reviews accolrding to whether they serve the principle of continuous improvement or whether they are stagnant (like Origin of Livestock Rule) or regressive (like allowing hydroponic production in organics). It’s important to finalize OOL and the Strengthening Organic Enforcement Rule as soon as possible. If supplements, etc. cannot be sustainable, they cannot be organic. The NOSB should establish their own work agenda.
Beth Dominick (Senior Inspector with QAI/on BOD for IOIA)
Human capital proposal: support comments from ACA and IOIA. Support 1:1 mentor program and grants to fund special projects (credentials study, workshops training, etc.). Shortage of inspectors could be helped by recruiting from outside the industry. Strategies to recruit—see IOIAs comments and increase funding! NOP could provide financial assistance with these projects.


