
Join The Cornucopia Institute as we keep you informed via live tweet and web updates from the National Organic Standards Board (NOSB) meeting in Seattle, WA April 24-26.
We will be sharing the play by play both below and with our Twitter followers, at #NOSB or by simply following our stream.
For background on issues up for discussion at the meeting, see:
- Cornucopia’s formal written comments on oversight improvements to deter fraud, Control Union Certifications, and petitioned materials
Friday, April 26, 2019
4:53 PM PT: NOSB Work Agenda
Summary of the NOSB’s Fall work agenda (Harriet Behar):
Will go to vote
Paper pots
Fatty alcohols
Liquid fish products annotation – TBD
Many sunset materials will be up for vote in the fall.
Use of excluded methods and vaccines will probably go to a vote in Fall (TBD). There needs to be more research on commercial availability.
Marine materials will remain a discussion; they are hoping to have a panel.
Genetic transparency of seed — TBD.
Induced mutagenesis and embryo transfer — TBD
They will probably vote on embryo transfer.
Research priorities will be collated by the subcommittees and be presented.
Policy and Procedure Manual update will be up for vote.
Other business and closing remarks:
NOP Deputy Administrator, Jennifer Tucker, said thank you to NOSB chairperson Harriet Baher for running this meeting, and to the entire NOSB. She also thanked the public and the commenters; noting that their passion and dedication is truly inspiring.
END OF NOSB MEETING
4:42 PM PT: Deferred Proposals and Final Votes
Proposal: Strengthening the organic seed guidance April 2019 was deferred—vote needed to return it to subcommittee.
Emily Oakley asked the board if they think stakeholders would rather have them pass it as-is and not make any changes. She thinks the vast majority of public comment wanted this to be passed ASAP.
Lisa de Lima noted that she thinks the changes are fine, but some people were unhappy about 4.1.6.
NOP Deputy Administrator Jennifer Tucker commented that, based on the process, this would be a substantive change.
Harriet Behar indicated that they can explain the change in a cover letter, and the NOP can do what they wish. They can still express our opinion with 4.1.6 and move this forward.
Ashley Swaffar added that she would be fine voting on this as originally written.
Steve Ela made the motion to accept all changes to the NOP Guidance 5029, as described in the proposal.
14-yes, 0 no—motion for seed guidance passes.
Deferred Proposal
Collagen Gel
Asa Bradman asked how they could address the confusion and move ahead.
Harriet Behar indicated that they had discussed leaving the words “as petitioned” and, in a cover letter, state that it is only being petitioned for use as a casing. That’s what we reviewed.
Steve Ela said that he thinks that is fine. They just need to inform the NOP and have it listed for what the petitioned stated, not just plain collagen gel.
Asa Bradman made the motion to add collagen gel, as petitioned, to 205.606.
Vote: 11 yes, 3 abstain, 0 no—motion passes
End of Deferred Proposals/Final Votes
4:15 PM PT
Crops Subcommittee
2021 Sunset substance review continued
Magnesium sulfate
NOSB member Emily Oakley reported that people who commented were in support of maintaining annotation, saying it’s usually a rescue treatment. They asked about non-synthetic, but previous reviews showed it maybe not widely available and is expensive. It is a highly used product and is used by 100% of fruit tree growers in the Pacific Northwest. Again, it is not widely used without documented soil deficiency.
Hydrogen chloride
NOSB member Asa Bradman said the material is used on cotton seeds to delint the seed prior to planting. No groups were opposed to its re-listing. The National Organic Coalition and Beyond Pesticides are in support of re-listing it, but there are concerns about using a strong acid. Consensus is that it would be great to move away from this, but there is not a commercial alternative at this point.
Ash from Manure Burning
NOSB Chair Harriet Behar commented that ash from manure burning is used to supply nutrients or other benefits. It is on the prohibited list. No one noted this is in use or recommended removing from prohibited list.
Sodium fluoroaluminate
NOSB member Dan Mortensen indicated that the material has been prohibited since 1996 due to environmental toxicity. It is used as an insecticide. Comments recommended continuing to list this as a prohibited substance.
Fatty Alcohols
Comments came in on this, and NOSB member Steve Ela wants to recognize those commenters. NOSB member Jesse Buie indicated that they received approximately 30 comments on fatty alcohols and summarizes several. Included were certified organic farmers that grow tobacco and other certified organic crops in rotation, including organic sweet potatoes, soybeans, and corn. Controlling sucker growth results in more efficient use of fertilizers.
NOP Deputy Administrator Jennifer Tucker noted that the material is listed for the fall NOSB meeting as a discussion document if board wants to proceed to a proposal.
NOSB member Emily Oakley indicated that it would be helpful to hear from the farmers, and she wants to note the absence of other commenters.
Harriet Behar suggested that they look at a material, not whether we like the crop. Tobacco is a legally grown crop in the U.S., and fatty alcohols are being petitioned for that crop.
NOSB member Asa Bradman commented that tobacco is really a damaging crop. We are at a time where we have explosion of addiction in U.S. and it’s a national emergency. Tobacco is still addictive material and we have to acknowledge that out loud.
Tom Chapman acknowledged that, but also carbohydrates that go into alcohol. He noted that the NOSB has a set of criteria, and if we stray from that, we move toward a slippery slope.
Asa Bradman agreed.
Emily Oakley added that there is an additional nuance because tobacco is a prohibited natural.
Sue Baird told Emily that is valid, but they have to evaluate by criteria. She noted that these are 4th, 5th, and 6th generation farmers, and this is their livelihoods.
Rick Greenwood added that, if you’re a farmer and have land and water, you ought to be able to grow what’s legal.
Steve Ela added that tobacco is going to be grown, so he would rather see it grown organically.
3:56 PM PT
Crops Subcommittee
2021 Sunset substance review
Hydrogen Peroxide
NOSB member Jesse Buie summarized the material (two listings); information can be found in the meeting materials. He said that the substance is widely used as a disinfectant and bleaching agent. Essential oils have not been studied side-by-side with hydrogen peroxide. Comments from 2015 were overwhelmingly in support. The subcommittee had no objections to continued listing.
Soaps, ammonium
NOSB member Rick Greenwood summarized the material; information can be found in the meeting materials. The material is used primarily for large animal barriers. There are other things that can be used, with variable efficacy. Low toxicity.
Steve Ela noted the question of effectiveness.
Oils, horticultural
Two listings will be lumped together.
NOSB member Steve Ela summarized the material; the information can be found in the meeting materials. He noted that most everybody is in favor of them. One question they had was whether there were other types of oil and if they would work in place of these oils. They received a new TR on this, and there were some listed alternatives. Most comments said that the alternative oils are not effective. There was one request for an annotation concerning worker safety. They are able to use them in a specialized manner and in terms of worker protection standards. He doesn’t think that annotation is needed. The point of using oils is that they don’t tend to disrupt beneficial insect populations.
Pheromones
NOSB member Steve Ela summarized the material; information can be found in the meeting materials. Used on insect management. They are widely accepted and widely used. Most organic growers use them in some form.
NOS member Scott Rice commented that he thinks there is one sprayable pheromone on the market.
Ferric phosphate
NOSB member Dave Mortensen summarized the material; information can be found in the meeting materials. The material is used to kill slugs and snails, especially in reduced tillage crops. They composed four questions and got good feedback from CCOF and VOF farms. Many stated they used this for protection. Most feedback says it is being used in a pelleted form. When used in combination with a chelating agent, the activity of the compound increases by orders of magnitude on slugs. Unfortunately, this also increases their activity on earthworms and other beneficial organisms.
NOSB member Asa Bradman indicated that a vote on this would be wrong. If this is only available as a formulated product, that is what the NOSB should be reviewing.
NOSB member Steve Ela said that the problem is that they don’t review products.
NOSB member Emily Oakley added that if they were just reviewing this without the inerts, then this product wouldn’t be separated.
NOSB member Dave Mortensen commented that, if everyone is using it in a formulated product, then that’s how we vote on this. They have to be cognizant in how its used. The efficacy is much better.
Potassium bicarbonate
NOSB member Emily Oakley summarized the material; information can be found in the meeting materials. They asked if growers are using alternatives and what they were using it on. NOSB did get some feedback, explained that it is used later in the season, when alternatives might damage the crop. It is used in fireblight, which has limited tools.
3:36 PM PT:
Discussion Document
Paper (plant pots and other crop production aids)
Steve Ela commented that this came up at the last meeting, and the subcommittee is waiting for the technical report (TR).
Harriet Behar noted that they found numerous synthetic fibers being used in paper chain and Ellepots. Numerous growers continued to stress need, especially for the paper chain pots. The petitioner is working to replace synthetic fibers with hemp. An updated sample will be received this summer.
They have asked for a TR for synthetic fibers for paper as a crop production aid.
Tom Chapman asked if there are synthetic fibers like this in newspaper.
Harriet Behar answered that they didn’t really get into that, but looked at specific fibers.
Tom Chapman noted that there was a public comment about glues in newspaper. He asked if there is a similar synthetic petroleum-based product being put in the soil.
Harriet Behar noted that they tried to use the newspaper TR as best they could, but really couldn’t because of different polymers.
Steve Ela added that he had contact with the petitioner and Ellepot, and both working hard to get synthetics out. They are just not there yet. Rayon, for example, is similar to adding cellulose. They may not be too far apart, and relatively small percentages of rayon. Lower percentages may be doable.
Emily Oakley encouraged the board to look at the supplemental newspaper TR. That TR does good job and shows there are already a lot of unknowns in use. She does not want to see the board create a stricter listing than what’s already allowed. Theoretically, someone could go out and put cardboard all over the field and it could technically be allowed.
Harriet Behar added that there are many synthetic fibers, and they didn’t fell like they had enough technical background to outright allow them. They want to do due diligence.
Sue Baird commented that she appreciated their diligence. She asked if there was any discussion on how hemp being incorporated.
Steve Ela had a discussion with one manufacturer who noted that it will all be hemp in the future. They have changed the playing field just by having the discussion, and manufacturers are trying to work with them.
Dave Mortensen mentioned the whole notion of continuous improvement, during transplant. They are helping pest management that relies heavily on plastic. There is potential to minimize reliance on plastic.
Emily Oakley repeated her request for reference of the newspaper TR.
3:20 pm PT
Proposal: Strengthening the organic seed guidance April 2019
Harriet Behar commented that there were a few tweaks, which were all fine except for one area (4.1.6). The rule allows for sale of crop from non-organic planting stock. The guidance read that, if the same plant is the mother plant to a planting stock, it had to be managed organically for a year before it could be on the organic market. But if it was a crop they could sell that as organic (example: rosemary cuttings). So they removed that from the item, but it is in the proposal as an issue. It didn’t make sense to sell the crop as organic the moment it comes onto the farm when it takes planting stock a year. 4.1.6 would be the only change to the item that is up for the proposal.
Scott Rice noted that the intent of improving the guidance was to close something that seemed nonsensical in the regulation. Harriet removed this portion of the suggested changed and noted that the regulations should be re-visited with respect to crop versus stock.
Behar said that this is still an issue that needs addressing.
Ashley Swaffar asked if they are removing “or a vegetative crop” in this document.
Behar noted that they are removing ANY CHANGE to the current 4.1.6 in the current guidance.
Tom Chapman asked if they are no longer changing 4.1.6, what other changes are there?
Behar commented that there was a little wordsmithing, but mostly it was a cut and paste. Scott and Steve looked it over.
Steve Ela said that it does add that there is an inconsistency in the rule.
Behar noted that this is an update in the guidance, not a recommendation for a rule change. No other changes except making it clear they removed the changes to 4.1.6.
Ashley Swaffar asked if they could send this around so we can look at it before the vote.
This issue was tabled for the time being under unanimous consent.
3:03 PM PT:
Petition for two compounds:
Ammonium Citrate (AC) and Ammonium Gycinate (AG) ‘
Dave Moretensen summarized the materials, as can be found in the discussion document. He noted that alternatives and cultural management practices exist. Also indicated that the NOSB has decided these are chelating ingredients, synthetic reaction-vessel. They asked for additional feedback and got mixed feedback in public comment. Some felt it would be good for the toolbox, while others thought we had enough tools and management practices already. There is weak supporting data in this application. Subcommittee was not supportive of this petition.
Emily Oakley commented that the reason the petitioner asked for this material is the Australian body deferred to the U.S. for its approval. She asked Clarissa (NOP) what was in the TR.
Clarissa (NOP) noted that there were concerns about nomenclature.
Harriet Behar added that they did have many robust discussions. They gave it an open-minded review and did not come to a positive decision.
Steve Ela commented that it gives him confidence because of the experience on the NOSB. There are other chelates out there that are available in organic. He struggles with essentiality and is ready to move it to a vote.
Motion to classify AC as synthetic
Vote: 14 yes, 0 no; motion passes
Listing to add AC as petition to 205.601
Vote: 0-yes, 14 no; motion fails.
Motion to classify AG as synthetic
Vote: 14 yes, 0 no; motion passes.
Listing motion to add AG as petitioned to 206.601
Vote: 0-yes, 14 no; motion fails.
Calcium acetate—petitioned
Steve Ela gave a summary of the petitioned material, information that can be found in the meeting materials. He noted that there were a limited number of public comments. One group was in favor, others against. There were no comments from growers on the record. Some commenters noted calcium foliar sprays are necessary in the growing season, but there are already some on the market. With sunscald from the use of plastic mulch, there were comments saying that allowing one synthetic to deal with the problems of another synthetic is problematic.
Ela continued that he had issues with essentiality. He questioned if they want to add another synthetic if there are natural materials on the list.
Emily Oakley commented that there are ample calcium products and methods to address calcium in crops. There are products and cultural methods. With sunscald, there are many cultural methods that can address this. She doesn’t think this is an issue that warrants the use of this synthetic.
Tom Chapman noted that there have been two comment periods on this material. The petitioner has had ample time to get those comments.
Rick Greenwood said that they have issues with sunscald with their avocados, and there are plenty of other materials available.
Motion to classify Calcium acetate as synthetic
Vote: 14 yes, 0 no; motion passes.
Motion to add Calcium acetate at 205.601
Vote: 0 yes, 14 no; motion fails
2:47 PM PT:
AITC (petitioned)
NOSB member Jesse Buie: Crops Subcommittee requested a TR (Feb 2018). AITC can have a short term deleterious effect on beneficial soil microorganisms and mutualistic fungal interactions which is observed for other broad-spectrum fumigants, such as methyl bromide and Telone II. This broad spectrum effect is not compatible with a system of sustainable agriculture. In addition, the availability of cultural methods or use of natural mustard plant cover crops precludes AITC from being essential to organic agriculture.
Motion to classify allyl isothiocyanate as synthetic:
14 yes; motion passes
Motion to add allyl isothiocyanate at 205.601:
14 no; motion fails
2:25 PM PT:
Hydrogen peroxide
NOSB member Asa Bradman: It is a common sanitizer and disinfectant with low toxicity.
Nutrient Vitamins and Minerals
NOSB member Tom Chapman: Used to add nutritional contents to food. Subcommittee will take all comments into consideration. Not going into detail now due to time constraints.
Peracetic acid
NOSB member Asa Bradman: Degrades rapidly and leaves little residue. Notes it is a respiratory irritant at eye-level. Beyond Pesticides’ report on this was helpful.
Potassium citrate
NOSB member Lisa de Lima: Public comment was supportive.
Potassium phosphate
NOSB member Tom Chapman: Annotated only for “made with organic.” Received very little comment on potassium phosphate. Received extensive comment from the trade association about many uses, but it is unclear whether the uses were for organic food products. Will seek further clarification.
Sodium acid pyrophosphate
NOSB member Scott Rice: Leavening agent mentioned by a number of certifiers on OSPs.
NOSB chair Harriet Behar: When this was first put on the list, there was concern that a multitude of phosphates would be added, but we haven’t seen that happen. The phosphates on the list seem to be working for the manufacturers.
Sodium citrate
NOSB member Lisa de Lima: Public comments were supportive of re-listing.
Tocopherols
NOSB member Asa Bradman: Used as an antioxidant and material to prevent rancidity. Currently on 205.605(b). Overall there is support for keeping tocopherols as necessary for processed food products. Comments indicate there are not enough non-synthetic sources available.
12:38 PM PT:
Biodegradable Mulch Film, update on research
Dr. Markus Flury, Washington State University
Soil physics and hydrology professor
Dr. Ramani Narayan, Michigan State University
Professor and elected fellow; is also technical advisor to many organizations
Dr. Flury: Here to speak on biodegradable mulches (BDM) and plastic mulches (PM) and biodegradable plastics (BP). Plastic is used a lot in agriculture. Most plastic is polyethylene plastic, which is not biodegradable and is very harmful to the environment.
In this experiment there were 2 field experiences. The degradation depends on the soil and climate, so we chose two different climates (cool vs. warm, different soils). We compared no plastic use to poly plastic in our experiments.
We monitor soil’s physical, biological, and chemical properties. Our research asked three questions: Does BP effect soil health? Does BP degrade completely in soil? Are residues released when BP degrades?
Soil health assessment chart:
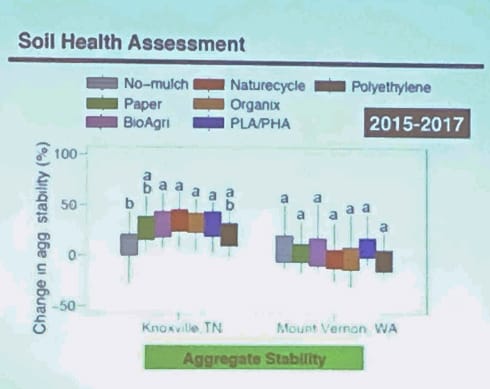
We see very little change in microbial communities affected by the treatment of plastics. But you see a lot of change in season (fall vs. spring) and they also change throughout the year. Very little change from BP.
BPM conclusions:
- Behave similarly to poly plastics (good substitute, therefore)
- No short term (two years) effect on soil health
- Seasonal changes are much more pronounced
- Soil health is a slow-changing process (so we need to be cautious)
Dr. Ramani Narayan: What does biodegradable really mean? Can microorganisms in the target disposal system (composting, soil, etc.) assimilate/utilize the carbon substrate as a food source completely and in a short defined time period? This is the basis. There is some confusion on this term—everything is biodegradable, so we need to be more careful in how we determine this. But in your system, you are defining it in a way that is is completely utilized, so that is the standard you have put into play in your document.
Another question is “biobased”—just makes the difference between where that carbon comes from (petro-fuel vs. plant biomass). 100% bio-based is a strict constraint that would never allow this program to move forward. Bio-carbon does have significant benefits, reduced carbon footprint, etc. (graphic).
NOSB chair Harriet Behar: You sent us a research paper, and you mentioned there is some use of genetic engineering (GE) to fracture/create that polymer.
Dr. Flury: Yes, some of these BP are made with GE.
Dr. Narayan: Yes, you can make these molecules using GE. You can create it without the use of GE, but you might see a huge price jump. Often the sugar comes from corn and corn is most likely GMO. Other sugar sources will be a dilemma. But the final product has no GMOs in it.
NOSB member Emily Oakley: You had two testing environments, but what about hot AND dry environments?
Dr. Flury: In hot and dry environments, you would expect less degradation. We expect more degradation in the warm and wet environment. So there are climatic differences.
NOSB member Emily Oakley: What’s present in the BDM that facilitates the biodegradation? What are the ingredients?
Dr. Narayan: Polyethylene is very strong and does not break up. If you use different linkages into the backbone of a long carbon chain, it allows it to break down better. The molecule is what is breaking down into smaller and smaller molecules. In order to make that film, there are other additives/ingredients that are added in small quantities—those additives will have to be GRAS or approved. Additives are needed to make it useable but I don’t think that is a sticking point.
NOSB member Dan Seitz: You can make biodegradeable and non-biodegradeable mulch out of oil; you can also make it from biomass/plants…but it sounds easier to make it out of the oil source?
Dr. Narayan: It can come from plant fermentation. But it will not happen overnight in the volumes you would want to use it.
Dr. Flury: Whether you make it bio-based or from oil is not that relevant. If you use corn, you use fuel/oil to make that corn anyway… so oil is a source anyway.
NOSB member Asa Bradman: In the research, was the mulch sitting on the top or disced in? You also mentioned the need for long-term studies.
Dr. Flury: The mulch was applied to the soil surface, but after the growing season it is rototilled into the ground so it doesn’t have to be removed. After you till the plastic, the organisms will chew up the plastic. At first there will be smaller and smaller pieces. What happens to those pieces before they are degraded? From our data, we know if will not happen in two years. In dry or arid climates, it might take 10 years. It’s not certain what will happen with these plastic pieces. For example, earthworms will eat them and we don’t know what the effect will be.
NOSB member Asa Bradman: With the impacts, are we concerned about a toxic or physical effect for larger organisms? There might be a portion that takes longer to break down, maybe that could get into the air?
Dr. Flury: One concern is soil erosion and leaching that could send those plastic pieces into the ocean before they completely degrade.
Dr. Narayan: The issue is that for biology to happen, water is needed. Dr. Flury’s work is necessary to confirm the lab-side study. There could be some biological effects on biodiversity, etc.
11:47 AM PT:
Perlite
NOSB member Scott Rice: Filter aid in food processing such as beer, wine, oils. Consistently supported by NOSB and stakeholders.
Potassium iodide
NOSB member Tom Chapman: Used as form of iodine in trace minerals substances and as sanitizing agent. Most comments around use as a dietary supplement and use in infant formula.
Yeast
NOSB member Steve Ela: Widely supported and widely used. Organic yeasts are available, but for certain uses they have not met needs.
Acid, Alginic
NOSB member Lisa de Lima: Derived from brown cold water seaweeds, used as emulsifier and other uses. TR reported no heavy metal residues in excess of EPA thresholds. TR pointed out alternatives, so if people prefer using alginic acid, they should submit comments before the fall meeting.
Activated charcoal
NOSB member Scott Rice: Only from vegetative sources; used as a filtering aid. Made from a large variety of sources. Allowed across international regulations. Wide support for re-listing from the community.
Ascorbic acid
NOSB member Scott Rice: Used as dietary supplement among other food processing uses; identical in molecular structure to the natural; humans must rely on dietary intake of Vitamin C.
NOSB member Steve Ela: We keep talking about fermentation. We will keep running into these processes and don’t want to get backed into corner where there is no other choice, like in the case of vaccines.
NOSB member Scott Rice: We may get some answers from the pending TR.
NOSB member Sue Baird: The TR for livestock vitamins noted that Vitamin C is commonly made with genetic engineering. Based on previous study, most of these vitamins are manufactured overseas. We could run into sourcing issues.
Calcium citrate
NOSB member Lisa de Lima: Public comment was supportive.
Ferrous Sulfate
NOSB member Tom Chapman: Use is primarily to address population based iron deficiency.
11:09 AM PT:
Jennifer Tucker (NOP): Regarding the last discussion, the public comment process to board proposals is very important for how the NOSB votes. If the board discussion yields proposed modification to a listing that changes how the public would have commented, that is considered a substantive change. If we have a substantive change that constrained public comment in some way, we would send it back to subcommittee. While non-substantive changes can happen in this setting, substantive changes would be problematic.
Acids, Citric
NOSB member Lisa de Lima: Produced through fermentation; used to control PH; used in gel formation; as stabilizer; as sanitizer, (mentions other uses)
No new information on harm to environment or human health.
Acids, Lactic
NOSB member Lisa de Lima: Produced through carbohydrate fermentation; uses are similar to citric acid.
No new info on harm to environment or human health.
NOSB chair Harriet Behar: Seen it used as a wash on meat products. There is an issue with whether its use takes a product out of 100% organic, as stated by a commenter.
Calcium Chloride
NOSB member Tom Chapman: Buffering agent, disinfectant (among other uses)
NOSB chair Harriet Behar: It’s used heavily in the cheese industry.
Dairy Cultures
NOSB member Steve Ela: Used a lot, most comments for re-listing.
Enzymes
NOSB member Steve Ela: Widely used, most comments support re-listing. Question of synthetic vs. non-synthetic is important, but it is annotated.
NOSB chair Harriet Behar: Certifiers do review that they are not from a product of genetic engineering.
L-Malic Acid
NOSB member Scott Rice: Significant comments on this. We have an updated TR received on March 20 and have not had opportunity to review it. Several certifiers recommended moving it to 605(b). Certifiers noted that they are verifying that it is L-malic acid and same caste number on National List. We will be reviewing the TR.
Magnesium sulfate
NOSB member Scott Rice: Wide variety of uses: tofu, personal care products, manufactured from several mineral forms. New TR received in April and that review will be forthcoming.
Microorganisms
NOSB member Steve Ela: Similar to dairy cultures; broader class. Some comments requested a clearer definition. Some commenting that there is a lack of clarity as to whether certain products made with assistance of microorganisms are covered. Otherwise, pretty broad support for relisting.
10:25 AM PT: Handling Subcommittee, petitioned materials continued
Pullulan (petitioned)
NOSB member Lisa de Lima summarizes the material (see discussion of petition in meeting materials). This material was allowed until the classification of the material changed (re-classified as non-agricultural substance). There are no other approved vegetarian supplements allowed.
TR found low environmental risk. Most public comments in support of pullulan were from manufacturer groups.
NOSB chair Harriet Behar: This seems innocuous. As long as we have a clear annotation on the vote.
NOSB member Emily Oakley: Eager to see the organic form developed.
Motion for pullulan to be classified as non-agricultural and non-synthetic
Vote: 14 Yes, 0 abstain, 0 no; motion passes
NOSB member Tom Chapman: Given concern about how the motion was written, we are going to make a motion to amend the listing motion. I move to amend it to include “for use only in tablets and capsules for dietary supplements labeled ‘made with organic (specified ingredients or food group(s)’”. Motion to change to this language.
Vote: 14 yes, 0 no; motion passes
Motion to add pullulan as petitioned at Sec. 205.605(a):
Vote: 14 yes, 0 no—motion passes.
Collagen Gel (casing)—petitioned
NOSB member Asa Bradman: Essentially this is an alternative for intestinal casings, primarily made from protein, commonly animal skins. Cellulose powder is also an ingredient here; when discussed in subcommittee, we thought that was more than an ancillary ingredient. It provides a mechanism to create kosher and other sausage products more efficiently and extends the market for organically produced material. Collagen itself is similar to gelatin that is on the National List. No toxicity issues.
We had a lot of discussion as to whether it is synthetic or not. We decided to list it at 606 as non-synthetic, but we struggled with that. Placing it on 606, there is a preference for organically-sourced material. The subcommittee voted unanimously to list this material.
There had been extensive public comment, primarily from membership groups, concerning what they consider violations of organic integrity. Concerns about the cellulose. It may be an issue because the collagen may have been from animals raised in conventional CAFOs, and that has been raised in public comments. In general, public comments are NOT in favor of lifting this. There is also concern it will stifle the production of organic source material for a product like this.
NOSB member Lisa de Lima: I don’t like where the material is sourced from; but do we wait for availability of organic product or do we take more of that share away from the conventional marketplace?
NOSB chair Harriet Behar: The petitioner indicated problems in storing for a long time when I asked whether they could save up enough of the organic source material and do a production run.
NOSB member Tom Chapman: Collagen is a pre-cursor to gelatin. All of the concerns also apply to gelatin. 606 is the right home for this material. I strongly disagree that placing items on 606 slows down the creation of an organic industry that can support this because of the certifiers requiring “organic first” in the supply chain checks. That could be a good opportunity for someone to make a lot of money in the organic market and increase competition.
NOSB member Asa Bradman: There is a difference here when compared to celery, because it’s a byproduct. It’s not being produced FOR organic like celery powder.
NOSB member Dan Seitz: The sourcing is a problem. From a consumer standpoint there is a real question. It’s not always good to speed up production when using a suspect approach. But it’s a good point that gelatin is already approved.
NOSB member Sue Baird: I agree that this should be on 606. You still have to do a commercial availability search before you can use it. By putting it on 606, you limit the producer-processor to doing a commercial search. This could actually build the market for organic.
NOSB member Tom Chapman: I hear the concerns about CAFO operations—but this won’t make or break them. If that’s the concern, we should look at adding manure to 205.602 as well if it comes from a CAFO. This question goes back to the farm level.
NOSB member Emily Oakley: We could add many materials to that (bone meal, blood meal, etc.), but it does not decrease the importance of discussing these issues. I echo some of Dan’s comments on consumer expectations.
NOSB member Steve Ela: Speaking about casings though, sausages are already encompassed in a conventional byproduct.
Motion to classify collagen gel as agricultural
Vote: 14 yes, 0 no—motion passes
NOSB member Asa Bradman: Now we are going to vote on the motion to add. There is a slight wording change to the motion with a change to annotation wording: “enrobement of meat products like a casing” (removed language “as petitioned”) at 205.606. The original petition said “sausage.”
NOSB member Tom Chapman: Why do we need this annotation, what’s the concern?
NOSB chair Harriet Behar: Without this we would be approving it as an ingredient in other foods and this is all we have reviewed it for.
NOSB member Emily Oakley: Going forward, should we always list the specific reason it’s petitioned?
NOSB chair Harriet Behar: Yes, its better for us to be clear what we are voting on.
NOSB member Tom Chapman: I find this to be very different than the pullulan petition because they specifically asked for it to be restricted that way. But here, the petition did not ask it to be restricted to that specific use. If we are going to restrict it, we should have had the public comment on that.
NOSB chair Harriet Behar: I don’t think “as petitioned” is clear enough for the program.
NOSB member Tom Chapman: I prefer the greater specificity in language. “As petitioned” might be hard to find the specific uses. This is the application we reviewed it for; and it’s what the petitioner asked for. There is potential this could be used in other ways.
NOSB member Emily Oakley: If we had the language “as petitioned” then they would see in the petition it was for this enrobement for meat process.
NOSB member Tom Chapman: But the request did not include that language.
NOSB chair Harriet Behar: I think this is what they discussed. If it was just going to be added without annotation, it should go back to subcommittee.
NOSB member Steve Ela: I am comfortable with this because this was as petitioned (the described use). But I think Tom makes a good point for the future.
NOSB member Asa Bradman: If we vote on this for this use, which I think is as petitioned, someone can always petition for another use.
NOSB member Tom Chapman: For me it’s a question of process. We don’t have this restriction on the casings material.
NOSB chair Harriet Behar: I don’t like the wording “as petitioned”—we don’t know if the NOP would have had this as the annotation if they looked at it.
NOSB member Tom Chapman: Would you support an amendment that just strikes the words “as petitioned”?
NOSB chair Harriet Behar: I am uncomfortable with that because we didn’t discuss other uses.
NOSB member Tom Chapman: But we don’t review annotations at sunset.
Paul Lewis (NOP): I appreciate that Tom is bringing up whether the public is aware of the scope, if we are going to be looking at annotations in the future. You may want to pause and discuss this further.
Move to table this to deferred items. No objections.
9:32 AM PT:
Voting to classify silver dihydrogen citrate as synthetic. Unanimous yes.
Motion to add silver dihydrogen citrate as petitioned:
13 vote no. 1 abstention (given no opportunity to annotate). Motion fails.
9:27 AM PT:
Silver Dihydrogen Citrate
NOSB member Tom Chapman: PureBioscience is petitioning to add the material to the National List. Petition received in Jan 2017 and amended twice. Technical review was received in May 2018. We considered this proposal in fall 2018, but sent back for review. Comments received this year were similar to last year.
The NOSB finds merit for this material, particularly around the need for alternative sanitizers in organic processing and its relative minimal potential human health impacts. However, concerns linger around its potential impact on the environment, its compatibility with organic production given the concerns around nano-particles, and surrounding the use of sodium laurel sulfate in the formulation. At this time, the Subcommittee is not recommending this material for inclusion on the National List. If the noted concerns can be mitigated or addressed, then the above material could be re-petitioned for reconsideration.
Three major concerns with regard to mitigation: (1) location of use (2) impact on environment (3) compatibility with organic production.
Chapman reports his opinion on this material has changed and he would like the subcommittee to consider it further to see if acceptable for use for some or all scopes petitioned for.
NOSB member Steve Ela: Expresses concerns about the quality of treatment systems across the nation, particularly in rural areas.
NOSB member Emily Oakley: How do you grapple with public comments and conflicting information? I echo Steve’s concerns about rural areas.
NOSB member Steve Ela: I don’t think there is a great answer to that. I err on side of conservatism and the precautionary principle and not putting this on the list. The only way to sort out conflicting public comments is through our own knowledge base.
NOSB member Rick Greenwood: Silver isn’t used that much. Expresses concern about silver in environment and sludge, as well as bioaccumulation.
NOSB member Dan Seitz: Expresses need for caution, given it takes 2/3 vote to remove from list once it is on. Also encourages caution where nanotechnology is an issue.
NOSB chair Harriet Behar: If we have to keep narrowing the use because we have a concern, there is more chance for abuse once it’s on the list. There was compelling info on resistance.
NOSB member Tom Chapman: What could the petitioner do to mitigate further concerns?
NOSB member Scott Rice: More information on nano issue would be helpful.
NOSB member Emily Oakley: Struggles with the vastly conflicting info on nanoparticles and effect on human health. Also, is there a question of residue and residual effects.
NOSB member Jesse Buie: Urban vs. rural disposal is an issue.
NOSB member Tom Chapman: Moves to refer silver dihydrogen citrate back to subcommittee.
5 yes, 9 no, motion fails
8:35 AM PT: NOSB continues looking at sunset materials.
Magnesium sulfate
NOSB member Ashley Swaffar summarizes the material.
Did not receive comment opposing the listing.
Parasiticide (Fenbendazole)
NOSB member Sue Baird summarizes the material (can be seen in discussion document). Annotation/wording was changed from what was in the discussion document, although the intent remains (changed to “fiber animals”).
Parasiticide (Moxidectin)
NOSB member Sue Baird summarizes the material (can be seen in discussion document). It is used in rotation. Internal parasiticide. (Notes ivermectin has been taken off the list.) The same wording change was made as in fendebdazole.
Comments from public on parasiticides were all favorable. Farmers say they are critical to have, especially in fiber-bearing animals.
Peroxyacetic/peracetic acid
NOSB member Jesse Buie summarizes the material (can be seen in discussion document).
No other discussion or commentary.
Xylazine
NOSB member Dan Seitz summarizes the material and its history (can be seen in discussion document).
Comments are in favor; not widely used. No comments recommended removal. Beyond Pesticides noted disparity between FDA regulations and what is allowed in organic—but did not see that mentioned in the TR.
Trace minerals
NOSB member Scott Rice summarizes the material (can be seen in discussion document). Mineral pre-mixed feed additives are widely used to ensure adequate intake. Manufacture varies because it is a broad listing.
NOSB member Dave Mortensen: Do we know how this effected animal weight gain and health, etc.? You would need efficacy data to determine if the pasture provided enough in terms of vitamins.
NOSB member Scott Rice: Comments did not point to efficacy, but more in relation to the pasture, etc.
Vitamins
NOSB member Sue Baird summarizes the material (can be seen in discussion document). They are listed by AFCO (the controlling body for all livestock feed). Clear we need these, but it was determined that some vitamins are being made from fermentation-production which uses genetic modification. In the past that was accepted. The TR said that genetic modification was commonly used in a whole list of vitamins, so NOP published guidance that instructed certifiers to watch for vitamins produced through those excluded methods.
NOSB chair Harriet: Some certifiers are questioning the source of some vitamins?
NOSB member Sue Baird: Yes, they were instructed by the NOP to question where they were coming from. We didn’t get any response as to whether they have to prove they searched for those produced without use of excluded methods.
NOSB member (unidentified): Non-GMO affidavits are used as part of confirming these kinds of searches.
NOSB member Ashley Swaffar: We did receive comment that the guidance says they “should” but not that they “must” verify. Just to clarify that language, it does not seem that they have to do anything.
NOSB member Dave Mortensen: This TR seems to indicate that all vitamins are produced by fermentation?
NOSB member Sue Baird: I think the TR shows the fermentation is the most common. Some are created by partial chemical synthesis. According to NOP’s direction, there should be review of that—assuming that there are vitamins that could be made without excluded methods.
END livestock subcommittee material review.
Thursday, April 25, 2019
6:05 PM PT: Livestock Subcommittee
Discussion document: Use of excluded method vaccines in organic livestock production 2021 Sunset substances review
Scott Rice, leading this discussion.
Harriet Behar commented that this discussion had numerous discussion lines and suggestions for what to do with vaccines. She asked how hard it would be to do commercial availability.
Option 1 was not popular; option 3 (commercial availability) had the most people approve it. HOWEVER they said they needed more help in finding what was commercially available. There was some discussion of “narrow and discreet allowance.” Many commenters needed this recourse to find non-GMO modified vaccines, and commented that there should be a phase-in period.
Ashley Swaffar noted that a huge problem is vaccine manufacturers don’t know if their products are produced with excluded methods. What if they say they don’t but they really do? That is a risk of option 3. She is in favor of option 2, allowing them as a class.
Harriet Behar noted that vaccine makers might be trying to grab the market.
Ashley Swaffar indicated that one company is claiming to have a salmonella vaccine produced without excluded methods, but she is pretty sure it does use excluded methods.
Paul Lewis (NOP) asked the question, “Is the broader issue of animal health acknowledged?”
Harriet Behar commented that they did include that in the document. They want it to be clear and consistent, all under the broader issue of animal health. They want vaccines to be acceptable and clarify the consistency.
Ashley Swaffar said that she doesn’t think option 1 is a good option. They would have to list vaccines as brands on the National List, and that would be a new precedent.
Harriet Behar commented that no one supported option 1.
Tom Chapman mentioned that he hears concerns about option 3, but struggles with why this is not the best way forward. The wording could be refined.
Ashley Swaffar thinks a lot of the vaccine manufacturers will list confidentiality in the manufacture processes. That might cause issues. They do give that information to USDA’s Animal and Plant Health Inspection Service (APHIS) though.
Sue Baird commented that she is for 2 or 3. Option 2 would be easiest. But if they do go with 2, it’s against the inherent intent of the law, no genetically engineered (GE) products allowed. Number 3 will be tougher, but they can give manufacture information to certifiers and have it under confidentiality. There should be specific lists available that let the producer know what is okay and what is not.
Scott Rice suggested moving on to 2020 sunset review since they are not voting on these at this meeting.
Atropine
Dan Seitz (lead) noted that it is approved for use in organic livestock. [Gives summary of the material, which can also be found in the meeting materials.]
There were only a few comments in favor of re-listing. It is not widely used but is considered essential for the rare times it is needed.
Dave Mortensen noted that this is a weird need to have. This is a nasty class of chemicals.
Scott Rice suggested that they only focus on the comments received.
Hydrogen peroxide
Jesse Buie (lead): [Gives summary of the material, which can also be found in the meeting materials.]
Iodine
Ashley Swaffar commented that there are two types of iodine; they will discuss together. It is widely used as teat dip and to treat wounds.
Iodine is a vital product. They did hear from commenters that they should annotate it to exclude MPs. They may be bringing forward an annotation on that, so they are going to take this back to subcommittee because they heard there might be issues with supply.
Asa Bradman commented on MPs. In California, MPs are on the list to be taken out. Probably small use, but something to discuss and there are formulations without MPs.
Emily Oakley asked if they would try and get that annotation done at the fall meeting.
Ashley Swaffar noted that they can’t annotate at sunset.
[NOSB agreed to discuss this further in subcommittee, where it would be more appropriate.]
Methionine
(skipping others temporarily because some stakeholders have to leave).
Heard about the continued need for methionine–it is extremely difficult to get from pasture alone. Methiomax was mentioned by Organic Valley. This product is made in Belgium and Organic Valley is trying to get approval from FDA. If it does work, the ingredients are 100% herbs. Birds would not need as much methionine from synthetic sources.
Emily Oakley said that she knows pasture producers that also use this material.
Ashley Swaffar commented that she spent so much time trying to get birds to go outside, and it’s difficult to get every bird outside everyday. There is a little bit of difference depending on flock size, but there are a lot of chickens that don’t want to go outside. It would be hard to lose this material because it’s hard to make it up outside. She is proud of the work the methionine task force has done. She is very passionate about this.
Scott said that they will pause on sunset review for today.
Adjourned for the day.
5:27 pm PT: Livestock Subcommittee
Scott Rice, Chairperson
Harriet Behar noted that they did not receive very many comments on oxalic acid. In Garth’s comments, he noted there are foreign producers. This material is allowed under the European Union (EU). Because of the lack of harmony between the U.S. and EU, he has seen several projects fail to achieve NOP certification due to confusion. Bee keepers prefer oxalic acid to vaporize in hive without opening it. The point was made in the proposal that it can be used in sugar syrup. As far as not having a standard, I don’t think we should punish organic bee keepers and not give them a material that they need.
Dave Mortensen said there was one subcommittee member who voted against it because there were no standards.
Ashley Swaffar indicated that she was the no vote, just based on no standards.
Emily Oakley added that, oddly enough, she finds herself conflicted as well and wonders why there aren’t more organic honey producers.
Harriet Behar commented that the NOSB did pass apiculture standards twice. Honey is concentrated flower nectar. Honey needed to come from land not treated with prohibited substances. Most bees will stay within 1.8 miles and the radius of non-treated land has been an issue.
As for why the NOP did not move forward with standards, things went dark after lots of calls from staff. She has heard it isn’t a priority, but this is important industry in Hawaii.
Jennifer Tucker (NOP) added that the NOP’s current priorities are import oversight, dairy, and origin of livestock.
Tom Chapman notes that in 2018 there were over 23,000 MT of honey imported into the U.S., most of it from Mexico. Honey is a large market when you run the numbers. Just because it’s not a wide production item in the U.S. doesn’t mean U.S. consumers shouldn’t have access to it.
Emily Oakley asked why there isn’t much organic honey production in U.S.
Harriet Behar commented that she believes it’s the forage zone. It’s a lot more labor not to use labor for mites, but there are certifiers certifying to their own standard, and she doesn’t know what every certifier’s standard is.
Tom Chapman said that in Brazil bees can forage 365 days a year in any one location. In U.S., it’s only while in bloom. Producers have to move forage zones, making it difficult to compete with international producers.
Harriet Behar noted that honey producers that sell locally don’t typically move hives.
Ashley Swaffar is going to keep a “no” vote because there are no standards.
Motions
•Classification Motion to add as oxalic acid dehydrate as synthetic:
14 yes
•Add to 205.603(b) as topical treatment external parasiticide or local anesthetic as applicable.
12 yes
2 abstain
Motion passes
5:12 PM CT: Certification, Accreditation, & Compliance Subcommittee (CACS)
Oversight Improvements to Deter Fraud
Sue Baird announced that CACS has compiled a document. They have had a lot of public comments and there has been a paneled assembled.
Harriet Behar commented that the Improvements to Deter Fraud Discussion Document was a way to summarize the import fraud panel and public comments. There was a lot of approval for the direction of this document. She has encouraged the NOP to review comments to target issues viewed as most important. Expansion of tariff codes were mentioned, and she encouraged the NOP to facilitate that activity.
Another point was that import certificates need to be tied to production. Some certifiers looked at what a producer had in stock and then could subtract the amount that had been sold, so some kind of system like this was suggested by one commenter. Another commenter suggested a pesticide residue database and acreage database. A lot of certifiers do collect this information.
There was some concern expressed that stop-sale authority might take too long, but they should start working on it now. She found product about to be sold and the farmer knew it was fraudulent. She contacted the certifier and the state certifier and informed them of what she found; neither of them could stop that sale. There are times when they really do need that authority. It was a couple of semi-loads of grain.
Tucker thanked the public for submitting comments. Most groups do have fraud as a top priority. This work has been ongoing. On tariff codes, they wouldn’t do it through rulemaking, but have recently updated memo with Customs and Border Patrol (CBP) and how they access information. The information does not have to be tied to a specific code and will have more visibility. Stop-sale would have to be a change in OFPA, and there are a lot of intricacies. They are working on alert systems so certifiers would be able to take action.
Tom asked Jennifer Tucker about the MOU.
NOP associate deputy administrator, Jennifer Tucker, commented that, right now, Agricultural Marketing Service (AMS) accesses data at a very specific code level. Now, they are organizing it by category and they get to see the entire category. It will be really useful once they have import certificates. This is going to take a long time, but is a huge domino game while stopping bad guys in the meantime.
Tom Chapman asked what is the next area of work for subcommittee.
Baird commented that there are many different facets to the fraud. Perhaps they might take bites at a time; they can’t address all at the same time. She asked Tucker if, in the absence of stop-sale authority, she could get agreement with another agency.
Tucker noted that they are having that conversation with Animal and Plant Health Inspection Service (APHIS), but they have their own regulations. She is excited about the working group’s potential to raise possibilities they don’t even know about.
Behar asked if the NOSB/subcommittee should wait to see what comes out of rulemaking and then take it from there.
Oakley commented that inviting Tucker to join the next call would be helpful.
Dave Mortensen responded to Tom’s question about next steps. It would be helpful for the board to get deeper into the weeds regarding what the data is telling them about how well they are doing. Some data can’t be revealed, but they are concerned that fraud is undercutting the price of grain. It would be really interesting during next meeting to see if they could get into outcomes.
Tom Chapman suggested two areas for the subcommittee to address: (1) a quarter of enforcement activities are handled by California State Organic Program. They should reach out to them. (2) The biggest shortcoming of the Organic Integrity Database (OID) is operations that are not listed. We need to encourage international partners to meet the standards. How do we fill information gaps in the OID?
Jennifer Tucker noted the subcommittee should talk about this more. There are several countries with no public registry and it would be good to create an open source program modeled after the OID that other countries could deploy in their own environments.
Tom Chapman suggested more consideration about the unique nature of fresh produce supply chains is needed.
4:28 PM CT: Materials Subcommittee (MS) Topics Discussion CONTINUED
Led By Emily Oakley (chairperson)
Discussion document: Genetic integrity transparency of seed grown on organic land
Harriet Behar stated that “Seed Purity” was the original name of this document. What are we trying to get to? Farmers, through no fault of their own, were having crop rejected, not knowing where the contamination came from. This rejection is a huge loss. And these are not new farmers. Many have not had this problem before. NOSB needs to know what is the integrity of that seed on a genetic level grown on organic land.
This is not only about organic seed in this country, there are international concerns. The NOSB is trying to set up a program to get that information to farmers. She has been speaking with the Organic Seed Alliance, and they will be reaching out to seed producers to see if a genetic transparency program is do-able, and legal, to test foundation seed from which hybrid seeds are made.
One cost is the availability of testing. It is also important to consider any unintended consequences if this project moves forward.
The committee hopes to move forward with proposal by this fall. They hope to make it more clear what is expected by each party in the chain, including non-organic seed producers. Non-organic seed producers have to be transparent about their seeds.
Comments were submitted:
- Numerous comments were submitted to remove the collection of data from the proposal;
- Make the testing of the seed strictly voluntary;
- Require no detectable levels of GE in any seed;
- Most commenters wanted it to be easier for farmers to figure out the level of contamination in their seed;
- In general, the public wants there to be more information from farmers before this moves forward.
- The discussion document elicited comments that should aid certifiers in figuring out what testing to do on seed and final crop as part of the guidance for this topic;
- Without transparency, farmers argue they will continue to lose access to markets.
Dave Mortensen commented that he gets the sense that the NOSB is afraid to know what the state of the seed supply is. He thinks they need to know with regard to purity. One suggestion was that they set up a group to conduct a study where people were sensitive to not smearing people in the process. Anything that is bee- or wind-pollinated in the grid; mixing conventional and organic has a risk of contamination. That group’s findings could then be shared with this community. He asked if this should remain in a discussion document or handled outside the discussion format. Farmers deserve to know what they are dealing with on the front-end.
Discussion document: Assessing cleaning and sanitation materials used in organic crop, livestock and handling
Harriet Behar stated that the goal was not to limit sanitizer use, but instead to better evaluate the petitions as they come to the NOSB to see where they fit in the constellation of sanitizers. They want to understand how they work, their differences, etc. “I do not hate sanitizers.” I understand about biofilms, etc. and how they can cause a food safety crisis.
She continued that they struggle to understand what is truly needed and what is not. Sanitizers kill biological life, and they hope this technical report will provide a reference. They want to know what ingredients are most compatible with OFPA. They agree this should be limited to those that have direct contact.
There was public comment asking for clarity on the use of these materials since there is no consistency among certifiers. Some allow 100% organic, and some do not.
Regarding the question of “uniqueness,” it was concerning the action and mode of action of the product, not the product itself. Multiple products may have that same mode of action. They would not accept just one option in a specific mode of action; they want to know what the mode of action is.
Ancillary ingredients have been used in the past for ingredients and excipients (anything except the active ingredient).
Steve Ela noted that stakeholders have asked the NOSB to make a comprehensive review of sanitizers for a long time and now, when they make this proposal, apparently OFPA criteria are perfect. Fundamentally, sanitizers go against OFPA, so they need to continue to figure out classes of use so they can rotate. He personally has some reservations; they don’t know what to ask for in a technical report at this point. He is tempted to take this process slowly.
Ashley Swaffar commented that the person that wanted the comprehensive review also wanted the NOP/NOSB to remove a lot of sanitizers. She has reservations about that. The produce industry always wants more options.
Tom Chapman asked if the technical report has gone out.
Paul Lewis answered. No, they are still trying to define the scope.
Tom Chapman suggested that maybe they can get some information from food safety experts. Stakeholders have disagreed on this issue and concept from the start. He thinks the subcommittee needs to consider ancillary substances more closely. There are conflicts on this that need to be addressed based on what was done in the past.
[Side discussion defining inerts and ancillary.]
Harriet noted that they are not going to be looking at ancillary substances in the TR.
Asa Bradman commented that comparative toxicity should be part of the criteria. And what the endpoints are.
Dave Mortensen said that it was never the intention that this was would be used to eliminate sanitizers already in use. They need a more systems-oriented approach to evaluating new things.
Emily Oakley noted that one comment asked to add the products of fermentation to the work plan.
BREAK
3:45 PM PT: Materials Subcommittee (MS) Topics Discussion
Led By Emily Oakley, chairperson
Proposal: Excluded methods determination April 2019
Harriet Behar comments that they are working through methods to determine if they should be excluded. There are many public comments saying that the language change regarding transposons developed using in vitro nucleic acid techniques is supported. This language was moved to the table of excluded methods from the notes section. Transposons created from other methods will be addressed later.
Vote: 14 yes vote, 0 no, 0 abstention/absence, 0 recusals
Motion to accept excluded methods determination for transposons.
Discussion Document: Excluded methods: induced mutagenesis and embryo transfer
Harriet Behar notes that there was some discussion and public comment/expertise.
They will hopefully have a proposal by fall on mutagenesis.
On embryo transfer, many certifiers weighed in, but they did not hear from a single grower. Most considered the use of hormones only in the donor animal allowable. The possibility of using the gene pool was not considered an issue, especially since this is already occurring with artificial insemination. There is some feeling that farmers should have access to this (on the female side). And that it would also be useful for a cow who cannot conceive. There is not much information regarding the young born or if there is an effect of the hormones. More research is needed.
There is some indication that the mammal may be affected. This method is not used regularly due to the cost and lack of need. This does not meet criteria for genetic engineering; however, the use of hormones made the Materials Subcommittee look at the proposal. The super-ovulation hormones given to a mother may change some genetics in the embryo. So, in a way, that might be considered a type of genetic engineering.
To the public: please continue to give us information on this issue.
Discussion document: Marine materials in organic crop production
Emily Oakley says that she hopes they can keep an open docket on this over the summer. There was a request for a task-force because it’s a complicated issue.
The big question is whether the NOSB is meeting the criteria of avoiding environmental harm. That is the goal.
Concerns:
- Precedent setting: is there a way to frame this as a wild crop instead?
- What about the ability to apply certification to this marine material? Certifiers may not have the expertise, though it is happening now.
Dave Mortensen said that panel experts speaking to the NOSB can be helpful. He wants to understand what certification processes are in place now and how they are working. The NOSB doesn’t usually hear from marine folks speaking about how changes in habitat alter the sustainability of fisheries.
Lisa de Lima noted that if they put something forward, it needs to be enforceable by OFPA. They should figure that out before starting a task force.
Scott Rice added that they need to learn more about the ecological questions concerning them to guide further work.
Steve Ela said that while good things are happening with company protocols, there are still bad actors, and he worries about climate change and other issues. The discussion document is a good starting point. He is possibly still in favor of wild-harvesting.
Harriet Behar commented that, in our rule, it states that we have to protect natural resources. Wild kelp is used in conventional, but organic cares more about the environment. Maybe with an annotation it would work. But organic certification would need ramping up. Maybe there could be a phase-in period. Also, do the harvesters mainly work for one company?
Rick Greenwood likes the idea of splitting the two panels. He wants more information about ocean ecology. We need to understand that before we can set standards. Maybe 10% harvest will destroy things.
Dave Mortensen added that how this plays out is very context-specific. It would be helpful to have somebody that knows about regulating what goes on in intertidal zone in the coast. If they are carefully managed in Canada and it is an unregulated process in New England, it’s a different set of concerns.
Asa Bradman noted that the status quo is not acceptable at this point. In some cases, the governments elsewhere may be promoting this for economic reasons, not considering the environment. This is basically taking nutrients from one ecosystem as pure inputs.
Tom Chapman agreed with Lisa regarding the need to figure out what authority the NOSB has to make standards first. He has a lot of concerns about the precedent-setting of certifying inputs. But he does not like referring to 3rd party standards. A panel is the best way to do it, but he struggles with priorities compared to other concerns. They have limited resources, maybe they should think about which panels are most urgent.
3:05 PM PT: Edward Brown, executive vice president of Pilgrim’s Market. Brown would like to thank the NOP for the Organic Integrity Database. He is concerned about the Origin of Livestock Rule and encouraged the NOP to adopt the final rule. He does a lot of private labeling and is noticing more and more stores using private labeling. He is concerned about fraud in this area. It is his understanding, under OFPA, that certifiers are accredited by the USDA and the USDA sets the rules. He is confused about the expansion of OFPA: doesn’t the USDA accredit the certifiers?
David Will, Chino Valley, CA Organic Program, and chair of Methionine Task Force. A UC Davis study on methionine was just completed. They are going to do an in-depth literature review and check on European uses and the law. Chino Valley’s goals are to fund more research. They will also look at Methiomax.
2:57 PM PT: Gabriel Flores As a Pure Bioscience distributor, Flores testified that he is here to plead with the NOSB for the Pure Bioscience product SDC to be certified. It sanitizes on a disinfectant level and does not have to be rinsed. Chlorine-based products create another problem because they begin to degrade and create harborage for more bacteria.
2:38 PM PT: Garth Kahl, farmer and inspector. Kahl testified about fraud and asked that the NOSB please use the sound and sensible principle. Right now, there is a super tanker called SS Fraud that threatens everything we’ve worked for. The amount of time spent inspecting operations is limited and it’s a zero sum game. He submitted that we need to stop and ask where the real threat is to organic integrity and act accordingly.
NOSB member Sue Baird: Where is the biggest risk?
Kahl: The biggest risk is fraud. Collaboration with control bodies is particularly important with imports. If there are fraudulent organics pouring in, our brand, our movement is swamped.
NOSB member Dan Seitz: There seem to be diverse practices around hydroponics and a split in community. Given hydroponic is a very different production system, wouldn’t we need standards for certifiers?
Kahl: Yes. We have equivalency with Canada, and they have detailed standards on container growing. Would that help solve the wounds of this split? I don’t know, but it’s a potential starting point.
2:26 PM PT: Jessica Walden, organic certifier at Quality Assurance International (QAI). She testified that nutrients, vitamins, and minerals are very complex, and the inconsistent application in organic products causes consumer confusion. QAI recommends implementing the January 2012 rule and creating a subcategory for ingredients used in infant formula.
2:19 PM PT: Miles McEvoy, Lacewing Auditing and Consulting, LLC and former deputy director of the NOP. Miles commented that the training system that is being launched is very important and that there is always room for improvement. He gave praise for the NOP and noted that we are lucky in the U.S., with respect to the robustness of the organic marketplace. We are ahead of the EU and Canada.
He also commented that the NOP has very little ability to amend standards and suggested that moving standards out of the USDA may be beneficial. All the standards are stuck behind USDA process. He recommend that the USDA have separate standards-setting authority.
NOSB member Sue Baird: What could the NOSB do to make this happen?
McEvoy: Suggested that the change would need to come through OFPA. Congress would need to make that change, but the NOSB could make a recommendation that would start the community thinking about it. It is a long-term concept that should be discussed and come to consensus.
NOSB member Asa Bradman: Concerned out accountability, who would make those decisions, and how it would function independent of the political system we have.
McEvoy: The NOP/NOSB should explore several different models, both private and federal. There would still be politics involved, but standards could be established which the community wants, and they would not be repealed, like OLPP or the delay with Origin of Livestock.
NOSB member Steve Ela: Asked for other examples of groups that would have federal rulemaking capability without directly residing in the agency.
McEvoy: A study needs to happen. Federal Trade Commission would be something to look at. Worthwhile looking at what those options are and if there is a better model.
NOSB member Emily Oakley: Is there ever a conflict of interpreting the standards within the USDA and then enforcing them? Or would that move to the standards body?
McEvoy: Suggested looking at Canada as an example of a possible structure. The government in Canada is responsible for enforcement of the certifiers. The standard-setting part of how they do things is something to look at.
NOSB member Rick Greenwood: Would this be funded by industry or federally?
McEvoy: Federal funding like the FTC, for example.
NOSB chair Harriet Behar: The NOSB is a young program in the USDA. Maybe that’s why they are playing catch-up on some standards. As the industry grows, there is need for more standards. At some point, the need for standard-making may not be as great as it is now. Also, she expressed concern that the Canadian model does not have a lot of public input.
McEvoy: Thinks it’s a discussion about how standards will be formed in the future. There is a problem here. NOSB makes recommendations and nothing happens, or they get repealed.
NOSB chair Harriet Behar: The NOP is responsive on materials, but other areas are suffering.
2:06 PM PT: Sandra Mays, senior associate and managing partner at Wolf, DiMatteo + Associates testified regarding cleaning and sanitation materials. She is confused as to the necessity of the sanitizer discussion document. She questioned the essentiality of creating a new framework for review when there are processes already in place. Why introduce EPA’s “safer choice” list?
The new criteria don’t seem to relate to the requirements of OFPA and the NOP. These materials are constantly changing, and certifiers review these materials whether or not they are on the National List.
The system isn’t broken. They recommend the NOSB does not continue with this process and discussion document.
In addition, adding pullulan to the National List allows the existing market to continue. Please accept this petition.
1:45 PM PT: Dave Carter, Crystal Springs Consulting, director of the National Bison Association, previous NOSB member, and consultant for Mercantile Health. He was happy the hear pet food regulations addressed at the beginning of meeting and noted that those standards need to be passed.
He says that Mercantile Health brought fenbendazole into the organic marketplace and testified that it is much safer and less harsh on the environment. Dave noted that parasitism may be weakest link in livestock organic production and that Mercantile Health had a fenbendazole product as an emergency treatment in organic laying flocks. They are submitting a petition on that soon.
Nathan Frizzell, director of operations at Full Measure Industries, testified that their company manufactures two products: calcium supplements and foliar applied calcium supplement and shade regulator. He noted that calcium is widely used and is important after K-P-N.
He testified in support of calcium acetate’s listing on the national list. It is being discussed as a synthetic, but it is a natural substance. He noted that the EPA has placed it on “safer chemical” list and that the other calcium supplements available in organic have serious limitations.
NOSB member Steve Ela: The biggest issue is that the NOSB has not heard from a single grower on this material. It is hard to document essentiality when they don’t hear from any grower and hard to say if people really want this when there is nothing in the docket.
Frizzell: Some organic watermelon growers have requested this product and he could get statements from them.
12:36 PM PT: Brian Baker, IFOAM President in North America, testified that fraud is a global issue, and he is pleased to see the USDA working with IOAS. IFOAM offers help in data sharing. We need to be aware of harmonization issues.
Baker says the international reputation of the USDA is undermined when it falls short of international norms. For example, hydroponics are not allowed under IFOAM standards.
Beth Unger of CROPP Cooperative testified that the final rule on Origin of Livestock is needed, and it has been frustrating to see the OLPP rule withdrawn. Unger is pleased the ACAs took this up and created a best practices guide.
12:28 PM PT: Dolana Blount of PURE Bioscience testified that her company is a petitioner for silver dihydrogen citrate (SDC). She claims this is not nanotechnology and that the concerns of resistance do not outweigh the benefits of its use.
She said one produce processor has been investigating the use of SDC on leafy greens. The results are being validated by government studies.
NOSB member Emily Oakley: Why do some processors hesitate to comment on this material?
Blount: There are corporate policies against endorsing one thing over another.
NOSB member Tom Chapman: Can you talk about concerns regarding antimicrobial resistance? And in the application for the manufacture of this, have you been contacted with concerns by the CDC or others in the medical community struggling with resistance?
Blount: Resistance is stemming through treatments to wounds, through bandages, using small amounts of silver over time—not a controlled use. Research shows it’s transient resistance, and when the silver is removed those genes do not remain prominent. SDC—silver ion is the primary ingredient, but it is coupled with citric acid.
NOSB member Tom Chapman: There are concerns about nano-materials. Is there some compromise language that could be recommended to mitigate that concern?
Blount: You assigned a particle size restraint for a technology that has no particles (because it’s ions). An annotation that says “is not nanotech and contains no particles” would be acceptable.
12:19 PM PT: Kyla Bedard, certification specialist for Vermont Organic Farmers, testified that she appreciates that paper pots were added to the work agenda. She says virgin paper should be included in the review as well as recycled paper.
Bedard says VOF supports the change to allow excluded methods in vaccines when others are not commercially available. VOF supports the implementation of the origin of livestock rule.
David Moore of Neudorff NA testified that the NOSB should continue the listing of ferric phosphate, given no new information to support de-listing. He said the single comment against it came from Beyond Pesticides, and they attributed zero evidence of causes of harm.
12:12 PM PT: Jackie DeMinter, Certification Policy Manager at MOSA, testified that about 500 MOSA members use vaccines. If verification of non-GMO status is going to be a requirement, we need resources to quickly make decisions and direct farmers to allowed products.
MOSA encourages passage or proposal for seeds at this meting. We like the strengthening of the guidance. Our written comments detail requests for clarification in specific areas.
We support the use of paper pots. We want clear guidance on the approval of new products that are similar to those we are already allowing.
Please move forward with a final rule on origin of livestock.
MOSA does not allow the rotating of cattle into the conventional sphere.
Winston Rost, Certification Specialist at Vermont Organic Farmers, testified that VOF supports genetic transparency for the seed proposal. This should be used as a tool; this would not be a burden to producers in Vermont (but they don’t produce a lot of corn).
12:05 PM PT: Paul Vandenberg of Paradisos del Sol Winery and Organic Vineyard testified that his primary reason for coming was to thank the NOSB and to encourage them to keep the standards high. CAFOs shouldn’t even be in your arena. Hydroponics are absolutely contrary to fundamentals of organics. He has just completed six years of zero inputs except water, manure, and labor–no pesticides of any sorts.
NOSB member Rick Greenwood: How do you control powdery mildew?
Vandenberg: I use canopy management with strong exposure to sunlight.
NOSB member Sue Baird: What is your average humidity?
Vandenberg: Choose the right thing to grow in the right place. I chose my farm location because I thought it possible to grow without inputs.
11:51 AM PT: Mary Chramiec, senior certification specialist at CCOF Certification Services, testified in support of the continued listing of vitamins and minerals, noting the annotation should be revised. She said CCOF supports option 2 for the proposed annotation. This option provides succinct and secure standards best aligned with the 2012 proposed rule from the NOP. It is the most likely to be consistently enforced by certifiers.
Regarding pullulan, 10 CCOF-certified operations use this substance in vegetarian capsules. We encourage the NOSB to suggest an annotation restricting its use to dietary supplements.
Dain Craver, organic grower at Royal Bluff Orchards, testifies that pheromones as insect mating disruptors are a major component in growing fruit. When we use these, we don’t have to spray. We get more ladybugs and diverse insects because of the pheromones.
He says it’s not 100% effective, but when it’s not, he uses allowed viruses, although resistance is causing him to use viruses at higher levels.
Craver asks the NOSB to keep all these products in use for fruit growers.
NOSB member Steve Ela: Are there other pheromone formulations?
Craver: They are working on it on the conventional side of things.
11:51 AM PT: Richard Mathews, WODPA policy consultant, and Robert Mensoides, WODPA’s new executive director, testify to the NOSB.
Mathews testifies it is time to complete the Origin of Livestock rule. There is no need for new analysis. He says once a herd has been converted to organic, all future animals must be organic from the last third of gestation. The structural error in regulatory text created a two track system. Lack of enforcement caused it to morph into a multi-track system. The rule must be brought in line with the preamble. The situation is killing small and medium sized dairy farms. We need the final rule today.
NOSB chair Harriet Behar: Would it be acceptable to have vaccines if we had a commercially available list?
Mathews: As long as farmers still have the tools needed to meet federal requirements and to make sure their animals are healthy.
11:41 AM PT: Anne Bikle (general public and science writer) based her comment on her book, The Hidden Half of Nature.
She is troubled by the NOP’s soil science, and offered the example of blueberries being grown in virgin “soil material,” allowing them to avoid the three-year transition period.
She says that covering soil with plastic suffocates the soil microbiome, the opposite of soil fertility as called for in OFPA. Why are organic farms headed in the same direction as conventional agriculture?
Bikle reports that soil-less media is thrown away after production, along with plastics, and is an environmental calamity. She experiences huge cognitive dissonance when it comes to the regulations regarding soil and what is being allowed. “Shall” and “must” mean REQUIRED. For the NOP and NOSB: please address the issue of long-term soil use.
11:36 AM PT: Julie Weisman of Elan Vanilla and Flavorganics LLC testified before the NOSB that only about half of organic products that use flavors are using certified organic flavors. This December the use of natural flavor will be used in organic products only if a certified organic product is not commercially available. The bar has been raised and certifiers and trade associations need to raise this awareness.
11:30 AM PT: John Hendrickson, farmer and paper pot supplier at Stone Circle Farm, testified that paper chain pots have had a huge impact in terms of profitability and viability for small farms. They reduce or eliminate the use of plastics, fossil fuels, and soil disturbance from weeds, while offering more productivity and keeping small diversified business in business by reducing labor.
A TR has been requested to review the materials in the paper pots. He hopes a vote will happen at the fall 2019 NOSB meeting. We want clear guidance on what will be allowed and not allowed (for fibers) in the paper pots so we can develop products that will work.
NOSB member Steve Ela: We need clarity on what fibers must be used in the pots. What is the minimum amount of synthetic fibers and what are they composed of in paper pots? Do we need plastics or can we use rayon, etc?
Hendrickson: The company is open to developing a pot without any of those synthetics to achieve a commercially viable product.
NOSB member Steve Ela: You’ve told us the help pot wasn’t commercially available yet. So what do we have to include in the vote, regarding synthetic fibers?
Hendrickson: I don’t need you to vote on ANY synthetic fibers.
NOSB member Emily Oakley: We can’t create an additional standard that is more restrictive than the current paper listing.
NOSB chair Harriet Behar: Will the development with the hemp be completed in mid-summer? We want an idea if that would be a viable option.
Hendrickson: I am encouraged by the booming interest in being allowed to grow hemp. We tested first samples last summer and it was not successful but a new batch is coming this summer.
Doug Currier of OMRI testifies to the NOSB that an annotation regarding marine materials may be appropriate, but there are many other substances where this might not work. Procurement methods may violate that the materials are not harmful to the environment but may fall outside the scope of certification.
A survey of the OMRI list was conducted, and some marine algae substances were found to be certified organic (formulated and non-formulated).
Regarding paper and other crop production aids, there are other paper production methods not mentioned in the discussion. OMRI has allowed newspaper and other recycled paper for use. OMRI’s rationale is that when applied to the soil, they will degrade and so meet the rules.
NOSB chair Harriet Behar: At some point I want to talk about coconut coir and buffering agents.
NOSB member Emily Oakley: If organic certification of marine algae is a viable option, how could the Organic Integrity Database be used as a verification means? There will be no ability to list the product as organic otherwise.
Currier: Marine algae is used as a crop input but is also certifiable, which is unusual for us. We would accept a certificate as verifying a crop input.
11:16 AM PT: David Hiltz, director of regulatory affairs at Acadian Seaplants LTD made comments to the NOSB on marine materials. He says Acadian is committed to harvesting activities being done in sustainable manner, and while they are not necessarily opposed to organic certification, they wonder about the precedent it may set. He says whichever standard is chosen needs to be clear and achievable, and a defined level of scrutiny must be identified in advance. Rock weed harvesting leaves the lower portion of the plant intact. In many jurisdictions there are limits regarding how much biomass is removed. Many publications show this is sustainable and dispute that rock weed is being over-harvested.
NOSB member Emily Oakley: I want to address the issue of precedent setting. We are looking at harvesting a plant from a wild native ecosystem. Any natural material allowed in organic should meet the same standards. Did you mention 10% of rock weed in Nova Scotia is harvested? Is this because it is hand harvested?
Hiltz: Harvesting of rock weed is not harming the environment. In Nova Scotia, all harvest is done by hand. Certainly by doing by hand gave more control, but mechanical harvesting is not necessarily harmful depending on how it’s done. The issue has more to do with governmental regulation.
NOSB member Steve Ela: I hear you’re doing a great job, but I am concerned with others.
Hiltz: Those organizations should encounter the same governmental regulations. This is a highly regulated industry. The future growth of our company depends on not destroying the resource.
NOSB chair Harriet Behar: When you talk about sustainable harvests, are you talking about regeneration of rock weed or others under review?
Hiltz: I am primarily focused on regeneration of rock weed, but I have looked at others and worked to understand other areas of fisheries. As long as terms are well defined and reasonable, we will meet them. We can’t be held to waiting until we have all of the science.
NOSB member Sue Baird: Do you have a matrix for percentage of how much you can harvest?
Hiltz: We use aerial photography and resource science team study estimates, and we work with local authorities to identify harvest limitations. We monitor what comes out of the sector, and once we hit the landing number, we will shift harvesting to a different area.
NOSB member Sue Baird: So it is already well-defined how much you can harvest?
Hiltz: It is in some areas–the exploitation rate depends on jurisdiction.
NOSB member Dan Seitz: What happens if multiple companies exploit the same area?
Hiltz: Companies shouldn’t harvest in the same sector in some places under licensing. They are trying to promote licensing for sectors to companies to control what’s being taken out.
NOSB member Emily Oakley: One of other commenters presented 25% in Nova Scotia. Some things are beyond our purview and left to local authorities.
Hiltz: We are not mowing down the entire coastline.
11:00 AM PT: Howard Whitney, principal scientist at Steen Research, LLC, testified to the NOSB that Steen has invented something that removes ammonia nitrogen from animal waste. We are seeking an organic input material label right now.
We oppose the petition to list synthetic ammonium citrate. The production of the synthetic compound contributes a huge amount of pollution. We can produce ammonium citrate at a price similar to fish emulsion. By 2020 we believe this natural material will be available at an economical price.
NOSB member Steve Ela: Will that be able to be used foliar and as a soil addition?
Whitney: Yes.
NOSB member Asa Bradman: Could you describe the process for production?
Whitney: We source from waste products from all forms of livestock agriculture. The process is business confidential. I am confident that this is a derived natural product. Any kind of animal manure will work.
NOSB member Asa Bradman: From a conventional large CAFO operation? Is there a scale?
Whitney: We’ve targeted a specific industry to get this launched, but we would like to apply it more broadly. We hope this will be a game-changing product. There is a lot of concern about the fish emulsion, and we believe our product could replace that competitively without removing it from the tool box.
10:37 AM PT: Alexis Randolph of QAI testified that QAI-certfied poultry operations are using paracetic acid. Where can we submit this information immediately?
NOSB member Emily Oakley: Please use the open docket to continue to submit more information on materials.
Rebekah Ritson of the Grain Millers told the NOSB it’s important to have better information in the imported commodities database. She said that verification across certification agencies is currently inconsistent.
Ritson is concerned about the organic industry alert system and the reporting system. Minor errors could cause a lot of business damage. We prefer to support “point 10” regarding the pesticide residue testing database. This information needs to be accessible to the industry as a whole. Certifiers would then have better knowledge for risk-based sampling. It would serve as a foundation for an effective risk-based alert system.
NOSB member Tom Chapman: Can you talk about your price fixing concern?
Ritson: We’ve heard this from producers from Canada. They say companies could determine from the data how much corn is available for a season and decide to only pay so much, depending on what’s available.
NOSB member Sue Baird: What do you think about the NOP being given stop-sale authority?
Ritson: We haven’t seen stop-sale authority exercised, even for conventional. It might take longer than we’d like, and it would require a lot of collaboration with state governments. If it can be implemented, that could be effective.
10:21 AM PT: Steve Walker, operations manager at MOSA testified that innovation moves faster than development of regulations. He said MOSA seeks counsel from other certifiers and the NOP when they encounter something new. He used to be frustrated when the NOP reported no policy on issues, but NOP Deputy Administrator Tucker relayed that this is the time for ACAs to make a decision. Walker values certifier autonomy and MOSA is currently collaborating on best practices for container production. He believes there are organic choices within these systems but needs the NOP to help develop regulations and to consider requirements for farm systems beyond the container.
Walker also called to move forth the origin of livestock final rule. Today our community is threatened. We need clear guidance and teeth.
10:06 AM PT: Harry Rice of the Global Organization for EPA and DHA Omega-3s (GOED), testified that GOED’s mission is to increase consumption of EPA and DHA. In regards to the re-listing of fish-oil on the list of non-organically produced materials, he said that organic fish oil does not exist and won’t exist until the NOP develops aquaculture standards.
Rice says fish should not be sourced from depleted or endangered fisheries. Fish oil must be sourced as a byproduct.
Gwendolyn Wyard of the OTA testified to the NOSB that the OTA wants to further restrict the use of pullulan in the “made with” category. Certified organic pullulan is underway.
Wyard said the proposal on genetic transparency of seed needs more work.
The clause on commercial availability of celery powder continues to present challenges because it’s at odds with organic demand. The supply chain needs to be built. The OTA is working on the supply chain and they hope to develop a model that will work for other materials on the National List as well.
Megan DeBates of the OTA testified to the NOSB that the 2018 Farm Bill provided $5 million to prevent organic fraud. She recommended the NOSB include how these funds should be spent in their discussions on the topic.
10:06 AM PT: Jay Kurtz of Devro discusses Devro’s petition for collagen gels. The addition of collagen gel will hopefully make organic sausages more widely available to organic consumers.
NOSB member Asa Bradman: I want to put this in the context of the larger discussion of 606. It is important to consider the long-term goal of ultimately having an organic source. I
NOSB member Steve Ela: If we approve this material, there won’t be as much incentive to develop an organic product line. We don’t want to delay development of an organic product.
Kurtz: Customers believe in transparency. Is this listing in-perpetuity? No. We want to see organic collagen gel.
[Cornucopia note: Because of changes to sunset voting, it has become very difficult to remove products from the National List once they are added. Read more on this issue.]
9:59 AM PT: Bjarne Pedersen is a consultant for the paper pot manufacturer Ellepot. He offered charts anbd graphs comparing plant fibers, and after 35 days cellulose is almost completely gone. After 35 days, the coffee filter was almost completely gone. Almost nothing happened to newspaper after 35 days. Because cellulose is expensive, newspapers use more glue which probably causes bad degradation. Ellepot wants to make sure product does go away when put in the soil.
NOSB member Steve Ela: What percent of (synthetic) plastic fibers do you think we should allow in paper pots? Would you use a percentage?
Pedersen: Ellepot tries to work with paper and plastic as 2 groups of materials. For many growers, paper needs to sustain certain strength and most cultures not ready within 15-20 days. We are looking for solutions to prolong life for two to three months.
NOSB member Emily Oakley: Was the newspaper in the trial glossy?
Pedersen: Non-glossy, which is why the results were surprising.
NOSB chair Harriet Behar: Have you tried hemp or flax in development of pots?
Pedersen: Yes, and we are bringing in more papers. He can’t disclose everything because of non-disclosure agreements.
9:49 AM PT: Kelly Pepper of Texas Organic Cotton Marketing Co-op testified that her organization has historically produced the majority of the organic cotton in the U.S. She asked the NOSB to renew hydrogen chloride, as it is needed for de-linting cotton seed. A mechanical de-linting process has not been found at this time, likely because there is not enough of a market yet.
Pepper appreciates the NOSB tackling the issue of GMO testing of planting seed. Contamination is a huge issue.
Alexander Strauch, DMV, a poultry veterinarian from Michigan, testified that methionine is the limiting amino acid for poultry. He said that decreased egg production and reduction in growth is an “environmentally hostile” practice with a higher environmental footprint. He welcomes alternative methionine supplies and agrees we should look at genetic selection, but there is no commercially-available alternative right now.
NOSB member Ashley Swaffar: We’ve heard about fish products as an alternative. Can you talk about that?
Strauch: Fish meal, other than not being allowed through “no animal byproduct” regulations, has some issues: short shelf life (salmonella risk) and unintended consequence of making eggs “fishy.”
NOSB member Scott Rice: The methionine panel reported there were no breeds that require less methionine to select.
Strauch: Right, I’m looking toward the future. The current turnover time for genetic selection and effects seen at market level is about four years.
NOSB member Dave Mortensen: How is the size of flocks being managed to contain the holistic management set?
Strauch: There are pros and cons. Smaller, pasture-raised flocks often have a higher parasite load. When given the choice, the bird may choose to poke its head outside and then stay inside. The ancestor evolved with thick forest cover (jungle fowl). Access to pasture in smaller operations is not one-size-fits-all.
NOSB member Sue Baird: Chickens are omnivores. If we can’t give them meat and bones, we need the methionine from another source, correct?
Strauch: Yes, they are opportunistic omnivores. The risks of meat or insect supplementation is an issue of food safety.
NOSB chair Harriet Behar: Methionine is a synthetic and we would prefer that most nutrition would come from products birds are consuming. Can you comment about MethioMax? I am familiar with all three of the herbs in it.
Strauch: I cannot find the same-structured peer-reviewed journal articles I would hold other nutritional supplements to. We also need to think about commercial availability. Is it a legitimate product?
NOSB chair Harriet Behar: I believe Organic Valley is working with the FDA to get it approved.
9:30 AM PT: Marisol Oviedo of the Northwest Horticultural Council told the NOSB that hydrogen peroxide is used by almost all organic fruit growers. She said organic agriculture needs access to multiple and effective sanitizers now and in the future.
9:26 AM PT: Dean Wesen, a dairy farmer at Wesen Organic Dairy, expressed concerns to the NOSB about the Origin of Livestock rule. Every time he comes to meetings, he hears the NOP is working on it. The rules need to be the same for all farmers. He appreciate the NOSB resolution on Origin of Livestock rule they passed last week.
He says the longer this drags out, we are losing many farmers.
NOSB chair Harriet Behar: Do you think the calves you raise on your farm from birth and go out on pasture for 6 months are healthier than cows raised in confinement and come back and have to learn how to graze?
Wesen: All cows love to graze and I think it’s bred into them. My cows do better later on in life, yes. ALso, beef is different from dairy. Part of the problem with dairy is the cows have been bred to stand behind a feed gate.
Kestrel Burcham, director of domestic policy at The Cornucopia Institute, urged the NOSB to uphold organic integrity. The loss of the OLPP was an absolute breakdown of public process. She shared concerns regarding lack of consistency in how rules are applied. There are two organic labels.
9:19 AM PT: Joanna Mirenda of the Organic Trade Association testified that the OTA represents over 9,000 businesses, and with only 22 days to comment, it’s a huge challenge. The OTA wasn’t able to fully engage on some topics, including marine materials. OTA was also unable to endorse one specific vaccine due to time constraints.
NOSB member Sue Baird: Regarding 205.236, origin of livestock, we have a problem with the ambiguity of (a), while (b) is pretty specific. Are we allowing baby cows on organic farms to be fed in hut, taken out of organic management, and brought back in?
Mirenda: We have heard of this. OTA disagrees with this practice and we galvanized our dairy producers to sign on to a letter to the USDA saying they disagree with the practice.
NOSB member Sue Baird: How does the NOP perceive this letter?
NOP Deputy Administrator Tucker: We agree there is inconsistent implementation of origin of livestock. OTA’s unified communication to the department as a priority has helped us in moving this rule along. Origin of livestock has been an area of contention for many years, and we hope to move as fast as possible in working through the legal process to gain clarity on this issue.
NOSB member Steve Ela: The reality is that it takes the NOP a while to vet things once they receive them. Any ideas?
Mirenda: We can look at the timeframe between when subcommittees submit proposals and we see it on our end. Maybe there is a way to make the process smoother.
Tucker: We have to give subcommittees time to work. By government standards the process is quite quick. When we have new people in the review process, it sometimes adds a few days to the timeline. We have gotten the timeline as short as we possibly can.
NOSB member Dave Mortensen: One solution would be to push the NOSB meeting later. When you’re reviewing things, it’s more like a two month turnaround.
Mirenda: 60 days would be ideal.
9:03 AM PT: Mike Crotser, certification manager at CROPP cooperative, supports the re-listing of celery powder. Over 25% of meat sales include celery powder, representing $9 million in sales in 2018. CROPP is committed to looking at potential substitutes, but there are none at this time.
Crotser also supports the re-listing fish oil and gelatin. Gelatin encapsulates fish oil.
NOSB chair Harriet Behar: In the Organic Valley public comments, you talk about using Methiomax, a product from Belgium. Do you have any information on how that product works?
Crotser: It is an herbal or plant-based product that increases the availability of methionine in the feed ration. Organic Valley can connect the NOSB with David Bruce before the fall meeting.
Rebecca Willows is senior compliance specialist for Organically Grown Co. and a member of OTA’s global supply chain integrity project. She supports NOSB’s move to require certification of the full supply chain. Rules regarding non-retail containers should be changed to require identification if “organic,” including contact info for the certifier of the final handler. Willows applaud the move to require certifiers to put information into the Organic Integrity Database (OID), as some certifiers do not update data until there is a complaint. The contents in OID are currently not reliable; there should be a consistent taxonomy.
8:34 AM PT: Mike Dill, food safety and compliance manager at Organic Produce Wholesalers Coalition (OPWC), told the NOSB that sanitizers are essential to their operations.
Dill says the concept of “uniqueness” for sanitizers is not supported by the Organic Foods Production Act (OFPA) and does not make sense. Developing new criteria for sanitizers may not work with OFPA. The new sanitizer that was petitioned is promising, but we are still in the discovery phase and are not ready to make a decision. We need the time to review and respond, and the review should be public.
We do not need additional criteria. The OFPA requirements are enough.
NOSB member Ashley Swaffar: You have a concern about uniqueness. Why wouldn’t one sanitizer work in your operation?
Dill: We would like to rotate more than we do. We have equipment rentals that require the use of specific sanitizers. We do not use any direct-contact sanitizers in our operation (no water use). It’s more important for our suppliers than for us. SDC is appealing to us because it has a different mode of action, but we are wary of the environmental impacts. We are hoping to postpone the SDC vote to evaluate the new information that came to light.
NOSB member Tom Chapman: Your use of sanitizers is environmentally based. Would a high level of good agricultural practices help avoid the use of sanitizers?
Dill: That would reduce the contamination of produce out in the field, so it would help prevent. But there is so much in the environment, you can’t account for everything. The biggest concern is post-harvest handling. For example, if you put a single contaminated head of lettuce in water with more lettuce, you can contaminate the whole batch. Post-harvest processing can make a small incident into a large food safety concern.
NOSB member Rick Greenwood: Have you seen resistance from the sanitation agents you’ve used? Do you do follow up with cultures?
Dill: I have not seen it and I don’t want to.
NOSB member Rick Greenwood: When you use a sanitizer that is that strong, you probably won’t have resistance. Low levels of antibiotics is more likely to cause resistance. Is resistance to sanitizers a real issue?
Dill: I don’t have an exact answer. We use our sanitizers in as low a concentration as possible. We try and follow best practices. When we have questions we look to our sanitation reps.
8:34 AM PT: NOSB chair Harriet Behar called the meeting to order.
Wednesday, April 24, 2019
6:13 PM PT: Laura Batcha of the Organic Trade Association (OTA) testified that there is a letter circulating that was sent to AMS about glyphosate and container production. The OTA membership felt like it was extremely important to hear a clarification from theNOP. There is an urgent need to determine how to apply practice standards to container systems.
She appreciates the need for complaints, but instructions don’t have to be coupled to a complaint process. She appreciates that the certifiers want to do the right thing, but if they’re not asking for land use history, then they don’t know what they don’t know. Instructions could get everybody on the same page and she looks forward to assisting with those instructions.
Batcha sees a lot of hemp production and organic has the opportunity to capture the market place. We want to drive this market to organic and discourage CBD around 606 (allowed nonorganics).
NOSB member Steve Ela to NOSB member Scott Rice: When you said that you talked to certifiers about glyphosate, did that cover spraying and putting plastic over it? What question was asked?
NOSB member Scott Rice: Spraying and then allowing production.
NOSB member Steve Ela: So even if plastic was laid down, it wouldn’t be allowed?
NOSB member Scott Rice: I don’t think conversation went to that; just that glyphosate would not be allowed.
NOSB member Asa Bradman: We need standards around container growing. CCOF did come up with a proposal, but there is a work agenda item to think about and develop standards.
Meeting adjourns for the evening, to resume Thursday, April 25.
5:54 PM PT: Pete Zambetti testified on behalf of Lonza Consumer Health and Nutrition. He is a representative for CapsaGel, a manufacturer of capsules.
Zambetti wants to add Pullulan to the National List. In 2004, CapsaGel submitted a petition and it wasn’t acted upon. In 2018 the full NOSB reviewed the petition. The subcommittee recommends adding Pullulan to the National List.
He claims there are not enough vegetarian options for the organic consumer. The OTA helped with the petition for this material if there are technical questions about Pullulan.
5:49 PM PT: Andrew, an organic dairy farmer from Washington state, testified that his family has been farming since 1989 and certified organic since 2004. He spoke about the Origin of Livestock issue and asked the NOP to take action.
5:45 PM PT: Christopher Peterson, of Cashton Farm Supply, testified to the NOSB that they are focused on organic and supply bulk chicken feeds to organic operations. They also sell bagged feed (usually for smaller farms).
They support the listing of synthetic methionine at the average rates currently allowed. Ammonia becomes a problem when producers over-feed protein. We do see 1,000 to 5,000 chicken barns that go out on pasture, and they still have ammonia problems. When the birds are 100% out on pasture they are usually smaller, and our season is short. So small-scale production does not exempt from these issues.
NOSB chair Harriet Behar: How are you working with the new methionine annotation? With the allowance over the lifetime of the bird?
Peterson: We are just starting with that; people are looking at it. Averaging based on the requirement of the bird.
NOSB chair Harriet Behar: So are you responding to their request?
Peterson: Yes, but they will have a breed spec that we can help them ration.
NOSB chair Harriet Behar: Certifiers are looking at how to determine if this has occurred.
Peterson: Most people will start by taking a percentage of that bird’s requirement.
NOSB member Emily Oakley: Positive example of continuous improvement.
5:38 PM PT: Colson’s testimony continues. He discussed glyphosate use with ACA colleagues and had dialogue with them. They consistently said they wouldn’t allow this.
NOSB member Scott Rice (USDA Accredited Certifying Agent): There is no case to dig into. It’s the mission and vision of ACA to have consistency in standards, and I saw that consistency quickly and loudly.
NOSB member Tom Chapman: A lot of production environments don’t have standards outlined (fungus, aquatic plant life, etc). Does MOFGA certify these?
Colson: Yes, there is inconsistency but that doesn’t necessarily negate the need for consistent standards.
NOSB member Dan Seitz: All of us in principle strive for consistency, but where there is such vast amount of information, complete consistency is not possible.
Colson: Farmers are innovative. We are asking for a moratorium or pause so we aren’t then catching up after the horse is out of barn. That’s where we are consistently finding ourselves. A pause or moratorium is worth considering
5:30 PM PT: Davis Colson of the Maine Organic Farmers and Gardeners Association (MOFGA) assists farms in production and marketing processes and he has a farm in Durham, Maine.
He testified to the NOSB that cultural methods are often overlooked in favor of inputs. Colson says these meetings are largely about what’s on the National List, but most organic growers he works with rely on cultural methods. Cultural innovation are the heart of organic farming, and he doesn’t want this to be lost in discussion over what products are on the National List. MOFGA supports a moratorium on hydroponic operations until such time as standards are developed.
NOSB member Emily Oakley: Please elaborate on hydroponic conversation.
Colson: He became aware of the hydroponic moratorium at the 2015 NOSB meeting in Vermont. Some certifiers have honored that. MOFGA does not certify container production.
5:22 PM PT: Kate Mendenhall, Director of the Organic Farmers Association (OFA) and organic farmer in NW Iowa, identified the top five priorities of organic farmers as import fraud, NOP enforcement, prohibiting hydroponic production in organics, the pasture rule, and organic dairy standards.
She states the NOSB should push/reaffirm their rule on Origin of Organic Livestock and the NOP should issue guidance on margins of pasture rule. She also encouraged a moratorium on hydroponic operations, and for the NOP to return this to the work agenda. The lack of uniform standards and implementation is hurting farmers nation-wide. Lack of clarity for the transition period is unacceptable.
NOSB member Emily Oakley: Can you elaborate on the three-year transition period clarity issue?
Mendenhall: Farmers need to call their certifier and receive a clear answer as to what is allowed and not allowed. This is not hypothetical; it is a direct question that requires a direct answer.
NOP Deputy Administrator Tucker: Glyphosate is not allowed. We have no specific case that is being talked about. We have learned about one operation and investigated and talked to that certifier. We need specific cases where we believe the rules have been broken and then we can evaluate against the regulations. We don’t have any evidence about these things happening on the ground and need more information. Give us a case and we will enforce the regulations.
Mendenhall: Hopefully it’s clear and will always be clear that prohibited substances will never be allowed. Otherwise it’s the end of organics. The real question is: if a prohibited substance is applied, can I certify my hydroponic production the next day? That kind of ambiguity leaves a lot of discontent.
5:10 PM PT: Jay Feldman, former NOSB member and Executive Director of Beyond Pesticides, tells the NOSB there are serious challenges.
We have to grow the organic market, ensure the public trust, and be transparent. Organic must support soil-based practices. IF the public doesn’t support organic, not much matters. Transparency is required to keep the public trust. Certifiers must act under uniform standards.
Feldman testified the NOP should be able to clearly articulate the law and the rules, and that has not been true for glyphosate.
He finds it painful to see lack of action on previous board decisions. He says hydroponic labeling does not need annotation.
5:08 PM PT: Leslie Touzeau, Material Review Specialist for certifier QCS, testified in support of the addition of fatty alcohols to the National List. Previously, many certifiers qualified fatty alcohols as non-synthetic; all farmers surveyed said fatty alcohols are essential for their production without an effective alternative. Vegetable oils do not adequately work, and suckering by hand requires much more additional labor. Labor costs would not allow producers to effectively hand-sucker their plants. Organic tobacco is essential to individual farm profitability and economic viability of the region.
QCS hopes the NOSB will recommend an extension of the use-up period.
NOSB member Steve Ela: Use-up means use up materials on hand. What does that mean for 2020?
Touzeau: We want that extension to mean farmers can purchase more materials for the 2020 season.
Abby Youngblood, Executive Director of the National Organic Coalition (NOC), is disturbed by the lack of consistency across certifiers.
We want the NOSB to set a moratorium on new hydroponic/container operations. We want more attention to peer review audits.
NOC supports adding energy infrastructure impacts to work agenda.
On excluded methods, especially induced mutagenesis, Youngblood says we need more input from stakeholders to figure out how much this is used in plant breeding. We are concerned about the possible chilling effect on organic breeding.
NOC opposes relisting of added nutrients unless they are required by other laws.
If we allow continued certification without standards in place for hydroponics, it is a slippery slope without clarity. We would ask certifiers to step up to that call as well, given the uncertainty.
Anne Ross of The Cornucopia Institute testified regarding import fraud. At the Fall 2017 NOSB meeting in Tucson, Cornucopia asked that the NOP act on fraudulent organic grain imports immediately. She gets calls from organic grain farmers asking about the progress frequently.
The Farm Bill and work done is good, but additional work is needed. She recommends the NOP get stop-sale authority.
When a foreign certifier has been penalized by other countries, it should immediately trigger investigation of that certifier and scrutiny of the products they certify. Weak penalties are no match for bad actors with deep pockets. Fines need to be meaningful or fraud will continue.
 Carol McRoberts and the Seattle Raging Grannies sang “the signature song” and the “restaurant that no one should eat in.”
Carol McRoberts and the Seattle Raging Grannies sang “the signature song” and the “restaurant that no one should eat in.”
They sang “GMOs have to go! NO GMOs.”
Fracking is very bad for the earth around it, and we think that some of the food grown is being served in restaurants…
“They won’t say what they are cooking today at the CHEMICAL RESTAURANT.”
4:49 PM PT: Eric Mandel, testifying for himself, notes he is also a PCC Seattle member. He asks the NOSB to consider public health in decisions they make. Most consumers assume organic is talking about farms, soil, and the whole system that produces food. Hydroponic produce should be labeled.
BPA has been de-emphasized and it’s a big issue.
NOSB member Emily Oakley to NOP: What is the status of BPA?
NOP Deputy Administrator Tucker: BPA has been de-prioritized on the work agenda due to a lot of critical topics being addressed, like imports.
4:45 PM PT: Kyla Smith of PCO Certified Organic commented on the crop subcommittee proposal on seed guidance and the sanitizer document.
She noted an issue with the term “vegetative crop” and the language in the seed guidance—the intent of this inclusion and the definition of this phrase is unclear. Vegetative crop does not met the definition of planting stock. It needs to be clarified what is and what is not allowed.
She requested inclusion regarding how or if inactive ingredients in cleaners and sanitizers should be reviewed. Certifiers are not reviewing these the same (some are reviewing them and some are not).
4:42 PM PT: Amanda Fulmer, University of Washington, testified in a personal capacity as a consumer and PCC member. She says “natural” ingredients should not be permitted in organic foods. Transparency and documentation are a few tenets of the organic movement. Natural ingredients are the black box of ingredient labels. Certain things that can be labeled as natural might not be appropriate for vegetarians or people with certain allergies. “Natural ingredients” may include sesame and not be appropriate for children with allergies.
NOSB member Lisa de Lima (Retailer): When you say “natural ingredients,” are you talking about flavors?
Fulmer: Yes, butter for example. Most organic butters now include natural flavor.
4:39 PM PT: Aimee Simpson, Director of Product Sustainability at PCC Community Markets, testifies that retailers are unique within the organic marketplace. PCC is the largest member-owned food co-op in the country. We are a certified organic retailer—we want to see organic grow, but not to the detriment of the integrity our consumers rely on.
The NOP must finalize the Origin of Livestock rule. We have already provided comment on the 2015 rule. Hydroponic production should not be certified organic. Soil matters even more with the issues of climate change. The Organic Foods Production Act (OFPA) was created to provide consistency, but the national label is unsure. The certifiers are applying different standards.
PCC gets questions from its customers all the time, including “how reliable is the organic label?”
Too often we are doing the work of the NOP, developing our own standards to fill the gaps. We ask you to push for the strongest and most consistent rules.
NOSB member Dan Sietz: Are you confident that consumers equate organic with in-ground production?
Simpson: Yes, we get questions daily. We frequently get comments saying people do not support “organic hydroponics,” a lot in relation to climate change. We are attempting to label the hydroponic produce, but that is difficult.
4:33 PM PT: Pryor Garnett of Garnett’s Red Prairie Farm grows certified organic grain in western Oregon. He discussed how organic agriculture is key in mitigating climate change. It reduces greenhouse gases, removes CO2 from atmosphere, and sequesters it in the soil. The NOP and NOSB need to act. Where plants are grown without soil, there is no carbon sequestration. Hydroponics are not organic. Hydroponic should not be eligible for organic certification.
4:30 PM PT: Jennie Cruse, Accredited Certifiers Association (ACA), represents accredited operations and organizations in the accreditation process.
We for the most part agree with genetic integrity of seed.
She comments on vaccines, saying that those being added to the National List does not make sense. ACA would support this if it was clear which vaccines were produced with excluded methods. We don’t want to support something that isn’t possible with respect to vaccines.
4:15 PM PT: Bob McGee of Straus Family Creamery tells the NOSB that he is representing nine family farms. When I asked our farmers what is the one thing you’d like me to convey to the NOP, it was around pasture standard enforcement and origin of livestock. I have heard changes in the industry require a second origin of livestock rule, and I can’t find what those changes are. Jenny [Tucker], it’s not clear what you’re asking. A new rule will take three to four years. Are we going to hear in 2022 and 2023 that we need to again go back to rulemaking? Many farmers don’t have two to three years to wait, which will lead to consolidation in the milk industry. Others will decide to take advantage of cost benefits. When we get to the new rule, will we hear the genie is out of bottle and there is no turning back? We need to bring forth the 2015 rule.
NOP Deputy Administrator Tucker: The key message from this meeting is the strong desire to move directly to a final rule instead of a second proposed rule. The data used in the 2015 proposed rule and the 2016 rule are different. The Office of Management and Business is concerned with changes in industry such as growth. We will take the message back; these decisions are made at an institutional level.
NOSB member Steve Ela: Jenny, you said that comments should be addressed to you or the program?
Tucker: I am concerned about legal changes that would prevent a rule from being implemented. A fair amount of agreement on the proposed rule could help us in making the argument that we should move to a final rule. This is not the NOP’s decision. There is no resistance from the NOP; we have to follow the rules.
NOSB member Dave Mortensen: The longer we wait, the larger farms get larger and it’s more difficult for medium and small farms.
4:07 PM PT: Albert Straus, the CEO of Straus Family Creamery, tells the NOSB that family farms are disappearing, as are our rural communities. Jenny Tucker said there is a change in organic dairy that we need to re-evaluate with OOL — that’s not correct; nothing has changed.
As a long term dairyman, I have offered to review the dairy training document.
With organic integrity at risk, retailers and brands need to work together: we are not accepting your milk if you don’t work toward organic integrity. We need to train certifiers to be consistent and we need to train inspectors. Look at the metrics dairymen can report that inspectors can verify: we don’t measure pasture as a crop. Dairymen also estimate what they are feeding their cows, but there are tools to get more accurate estimates.
NOSB chair Harriet Behar: You’ve put a lot of effort into value-added product, but a lot of farmers are reliant on a buyer.
Straus: I’ve had a half dozen farmers call saying they need someone to take their milk because of this.
4:01 PM PT: Kristy Badger, National Organic Coalition (NOC)
Kristy’s family lost their dairy farm this year.
During NOC’s pre-NOSB meeting, some said stakeholders feel pressure to agree to documents out of fear the documents will be pulled from the work agenda. Dr. Tucker represented this to her.
At yesterday’s NOC meeting, stakeholders said the NOP must address issues in hydroponic production. While there is large stakeholder agreement that these are priority issues, we are not in agreement to surrender our voice to the program.
Badger asserts that Origin of Livestock has not been a priority, and the NOP has decided its priorities outweigh those of existing organic farmers.
NOSB chair Harriet Behar: I understand the emotion seeing these beautiful farms go out of business.
NOSB member Emily Oakley: It’s frustrating to sit on this board and feel we don’t have the power.
3:59 PM PT: Ryan Mensonides, Providence Farms Mensonides, reads off statistics for his farm due to the problems in the dairy industry. He has lost a huge amount of money, and his family is personally at risk in many ways. He received a 30% pay cut this year, which puts them below cost of production. He and his children and wife will lose their farm.
Because the Origin of Livestock (OOL) rule did not get implemented, we have an oversupply of milk.
He shared a video of his farm and family, commenting this is what we are losing if the NOP doesn’t follow through with the OOL rule.
NOSB member Emily Oakley: We don’t have control over whether the OOL is implemented, so I wanted to give the NOP the opportunity to comment.
NOP Deputy Administrator Tucker: We are open to adding this to the agenda. We’ve heard comments about this being implemented before more commentary. If that is not possible, we will have second proposed rule for more commentary.
[Cornucopia comment: 10 years ago the previous administrator of the NOP declared that the rule was a priority and said it would be the next rule they would work on. This has been a complete and abysmal failure on the part of the NOP to get this done as they cowtow to the giant dairies benefiting from cheap replacement milk cows.]
Mensonides: If this is going to be a one to three year process, I am going to be out of business. There has to be a sense of urgency. This urgency has to go to your boss.
3:51 PM PT: Tony, along with and his fellow dairy farmer friend, Ryan, testified that they are dairy farmers who were certified organic in 2007 and were shipping to Horizon. Point of origin for livestock is being highly abused, making it difficult for us to keep farming. There are people and farms who raise calves in feed lots, breed the cows, and then convert them to “organic” by the time the cow comes into gestation. It is much more expensive to raise an organic calf than to raise a conventional calf. So we have an oversupply of milk.
Ryan left Horizon and went with Organic West, and I stayed with Horizon. They were bought out by Danone, and to make a long story short they canceled my contract last January 1. I’ve always been a member of a co-op and there were seven producers in the same situation and through transition. I sell what I can organically and the rest goes on the conventional market. If we implemented the rule in place and have certifiers do what they’re supposed to do, and it would eliminate the (milk) surplus. If you implement the point of origin law, the exodus of farmers from organic would stop.
3:48 PM PT: Thomas B Harding Jr., LVOG, Inc.
Harding represents the petitioner for fatty alcohols. He claims fatty alcohols are not just essential but important for worker health.
Without availability of fatty alcohols, organic tobacco will not be a viable crop. He estimated $23 million in losses in his region if this material is removed.
NOSB member Asa Bradman: Please clarify “health and welfare.”
Harding: When workers do it by hand, they are exposed to the tobacco leaf. This material helps the attendant sickness from occurring.
NOSB member Asa Bradman: So you are using “health and welfare” in terms of exposure to the tobacco, not more broadly.
NOSB member Emily Oakley: It sounded like there were some growers already using this material. Can you explain how this was being used before it was approved for 7+ years, as was discussed earlier?
Harding: Certifiers approved this material because it was classified as a natural fatty alcohol, not a synthetic, at first. They later decided it just crossed the border into a synthetic. And then we submitted the petition.
NOSB chair Harriet Behar: Would you prefer more discussion or an immediate vote?
Harding: Please go to a vote as soon as possible because the next season is in question.
3:38 PM PT: Peter Nell (CCOF)
Nell appreciates the discussion document on marine materials. CCOF does not support organic certification, under the wild crop standard, for marine materials. He recommends the NOSB use an expert panel to discuss at the fall NOSB meeting.
NOSB member Emily Oakley to NOP: Is a task force on marine materials feasible?
NOP Deputy Administrator Tucker: I am interested in learning what happens in the expert panel and we’ll take it from there.
NOSB member Asa Bradman: Peat moss and liquid fish products are also relevant to this conversation. We need to know the environmental impacts and think carefully with regard to the changing world.
Nell: CCOF’s fall comment included a section on whether all ocean-based inputs should be moving toward a third-party verification system. This could be further examined through a task force.
5:33 PM PT: Phil LaRocca, Owner of LaRocca Vineyard and Chair of CCOF Board
Phil was shocked to hear that a container grower can spray herbicide on the ground, put plastic on top, and grow inside a container.
The whole system should be certified organic. There will be glyphosate residue for many years down the road, even after pulling up plastic. We need to re-examine all the ways we use plastic, even in row-crops. It’s an issue: climate change, filling up landfills, etc. We should have standards for use of plastic.
NOSB member Emily Oakley: I agree. Question: you said you spoke with the CCOF Executive Director And they told you about this hydroponic/glyphosate issue?
Phil LaRocca: Yes, that conversation happened.
NOP Deputy Administrator Tucker: This is not a program policy or specific farm, this was a comment on a hypothetical. There are split operations, and the same kind of questions come up with them regarding land use histories.
LaRocca: You are saying there is no rule that states that you can’t put glyphosate and put plastic on top of it and certify the product organic?
Tucker: Glyphosate is not allowed in organic production. I refuse to engage in hypotheticals.
3:25 PM PT: Zea Sonnabend, CCOF
Sonnabend suggested genetic integrity of seed needs to be reworked to state a clear goal. She generally agrees with Kiki Hubbard’s comments but is interested to see how many would voluntarily disclose genetic testing results.
Dave: We have had genetically modified corn since 1998 and organic farmers are living in a matrix of 90 plus GM corn varieties grown around them. He feels a sense of urgency.
Sonnabend: Disclosing it in confidence to the inspector doesn’t necessarily have to be in public information.
NOSB chair Harriet Behar: The data collection was never intended to be public and seed companies still have to be reassured of that.
3:19 PM PT: Alan Lewis of Natural Grocers testified that Natural Grocers does not label [“organic”] hydroponic produce, and they don’t know whether it’s actually certified organic, grown in soil. They don’t know what to tell theirr consumers.
Natural Grocers employees are instructed to note whether incoming produce is potentially hydroponic (high risk produce types like tomatoes, berries, etc.), whether the brand is known to provide hydroponic produce, and whether the product’s certifier is known to certify hydroponic operations. If those three things are true, Natural Grocers employees mark them as such in-store. Not only are hydroponic practices undisclosed or proprietary, but many of the practices are unknown with no set standards.
He believes hydroponic produce needs to be labeled if the organic seal is going to remain intact.
NOSB member Dan Seitz: When discussing hydroponics previously, the question came up over whether you could have a supplemental label; is that allowable under organic regulations?
NOP Deputy Administrator Tucker responds that we support opt-in labeling, as long as the organic claim is truthful and the produce is certified.
NOSB member Dan Seitz: I know you would prefer an opt-in label, but could a segment be required to have an extra descriptor?
Tucker says that would require regulatory change to impose that label on someone.
NOSB member Tom Chapman: Looking at your comments from the Jacksonville meeting, you said you don’t support a hydroponic label.
Lewis: About two years ago [USDA Secretary] Sonny Perdue stated unequivocally hydroponic could be sold as organic. We don’t have a choice right now and neither do our consumers. There is now confusion between different scales and practices and inputs. We have basically created two brands under one seal!
NOSB member Asa Bradman: I agree. Labeling is one approach we should consider. And possibly different standards need to be discussed.
NOSB member Dave Mortensen also agrees.
3:08 PM PT:
Cathleen McCluskey, Organic Seed Alliance
Improving sourcing of organic seeds will lead to more investments in organic seed production and more varieties for organic farmers to choose from. She appreciates the additional language that clarifies non-compliance and equivalent organic varieties. She wants the NOP to pass the proposal.
3:08 PM PT:
Roland McReynolds, Executive Director of Carolina Farm Stewardship Association (CFSA)
McReynolds says he is here to support the petition for the use of fatty alcohols for sucker control in organic tobacco.
The majority of organic tobacco farms signed a document asking for a continued allowance. Mid-scale family farms are most affected—sucker control is critical for these farms.
Organic tobacco allows these farmers to accept greater risk for other crops. Thousands of acres have been converted to organic because of organic tobacco. Tobacco is grown in rotation with organic sweet potatoes and local cereal grains. These farmers will be faced with the decision to stay in organic production or not, depending on the continued use of fatty alcohols.
NOSB member Emily Oakley: My concern is the essentiality is based on economic need. Herbicides also make crops economically viable. Do you think economics is really a compelling reason to list an ingredient?
McReynolds: A technical evaluation report (TR) prepared in 2016 identifies that the evaluation criteria for synthetics is met. Toxicity is low, as is environmental contamination, according to TR. There are no human health risks and it is a benefit for worker safety (heat and green tobacco sickness). The TR demonstrates this meets the requirements for an addition to the list.
2:55 PM PT:
Lynn Coody, Organic Produce Wholesalers Coalition (OPWC)
OPWC has provided detailed comments on sunset materials. They agree with most recommendations for this meeting, but addressed several disagreements.
2:38 PM PT: Public comments begin.
Kiki Hubbard, Organic Seed Alliance (OSA)
OSA appreciates clarity on excluded methods and offered detailed recommendations in written comments. OSA is concerned about farmers being required to collect information. For example, organic field corn producers have limited choices. And concerns remain about implementing the pilot project outside of an expert body. OSA wants to support more transparency while encouraging investment in seed production and increasing availability of organic seed production.
NOSB member Harriet Behar: Looking at the discussion document from last fall, it does not stop any farmer from using any corn seed with any limit of contamination. If a farmer wanted buy a certain variety knowing it’s high or not high (in contamination), why would that stop them from using it–if not trying to get into a market that required less contamination?
Hubbard: She is not concerned about farmers feeling discouraged about using a particular variety. There needs to be a lot more information collected before pilot project, and she doesn’t want to establish a de facto threshold that might result in fewer organic seed options.
Hubbard claimed most seed companies are testing and often re-direct seeds that test high for contamination to the conventional market.
NOSB member Emily Oakley: How can we encourage farmers to ask for information?
Hubbard: That encouragement needs to come from the certifying and inspecting community. It is not wise to encourage this of all growers. She is advocating for a cautious approach.
2:21 PM PT: Dr. Burley summarized, insufficient methionine leads to health issues and right now there is no single strategy or ingredient to replace synthetic methionine in organic poultry diets. Her recommendation is to continue until an effective alternative can be identified.
NOSB member Harriet Behar: Will blending ingredients work? In the Organic Valley public comments, they talked about Methiomax which enhances methionine?
Dr. Burley: I did look into herbal alternatives and found issues. The product consisted of several herbs but did not list what those herbs were or what country they came from. She does not believe it is a strong alternative source.
NOSB member Harriet Behar: I was able to identify herbal ingredients in it.
NOSB member Emily Oakley: How many studies regarding pasture and forage?
Dr. Burley: It was a limited study by a masters student at Penn State. The pasture was not rotated, but she believes this would only make a minor difference.
Dr. Burley responded to an unheard question by NOSB member Ashley Swaffar: Laying hens produced less eggs and broilers took longer to grow so you can feed slightly lower levels on a daily basis (because they are not putting as much muscle on per day). In this case, it’s lower production trading off for lower methionine.
2:07 PM PT: An update on the methionine task force was given by:
Dr. Kristjian Bregendahl (Devenish Nutrition);
Dr. Heather Burley (McDaniel College); and
David Will (Chair of Task Force).
David Will: The task force formed in 2007. Industry group that represents 85% of organic layers from pastured to larger scale producers funded a high-methionine corn trial. UC Davis did a study on methionine via the black soldier fly, and we are funding a literature review. The allowed use of synthetic methionine has been a contentious issue at past meetings.
Dr. Bregendahl: Birds require amino acids. He makes laying hen diets, and asserts that, in order to remove added methionine from the feed, one would have to considerably increase soybean meal in the mix, potentially causing issues with extra protein. He said that methionine and lysine need to be in the appropriate ratio, and this is made more difficult when methionine is provided in an alternative way. Brazilian tree nut protein powder might work, but it is not commercially available. Methionine deficiency causes low egg production and egg weight, as well as animal welfare issues including poor feathering, aggressiveness, feather pulling, and cannibalism.
Dr. Burley: Methionine is one of 10 essential amino acids for poultry. Excess protein leads to animal welfare and human worker issues. Poultry fed a plant-based diet receive incomplete protein. Since organic birds are fed vegetarian diets, it’s hard to get amino acids.
Alternative breeds are being considered. Amino acids needs are different based on individual breeds within categories. Poultry categories (broilers, layers, turkey, etc.) differ in amino acids needs based on age, size, and purpose (build different proteins for meat vs. eggs).
Regarding alternative feeding strategies, lower dietary energy increases feed intake but not enough to make up for methionine deficient diets.
Pasture intake for birds is quite low–only 2-8% of diet. Foraging/Pasture access is not sufficient to replace synthetic methionine. Pasture is not available in cold/winter months. Pasture rotation is needed to prevent burn out from manure. Insect intake is very low (only .42% of crop contents).
12:25 PM PT: Discussion with the celery panel continues, with lunch to follow.
Logan Peterman (Organic Valley/CROPP): Maintains conventional celery powder is still essential, and claims it is the only ingredient that can fulfill safety requirements. Cured meat. Accounts for 25% of organic meat company (OMC) gross revenue, roughly $1.1 billion dollars in the organic meat market. Pork products are the biggest area where this is used.
Peterman identified the constraints as:
- Varietal differences, and the reasons varietal differences exist
- Fertility/regional variation
- Nitrate to nitrite conversion performance
- Within OMC, the sensory panel approval (taste, color, shelf-life) is also critical
He identified the following market dynamics:
- Large financial risk to farmers and processors (including risk of that product not meeting consumer demands).
- Product is used in very small amounts (amounts to ounces per hundred pounds—small but critical part of product).
- Potential gain for processors and farmers is limited (essentially asking farmers to adopt a crop that may not produce profit).
He says the conversation bears heavily on a number of products listed in § 205.606 (Nonorganically produced agricultural products allowed as ingredients in or on processed products labeled as “organic”). How do products that don’t represent a large enough opportunity for the market develop other opportunities?
He also inquires about the potential environmental impacts if the use of synthetic nitrogen is increased in conventional celery production.
NOSB member Harriet Behar: Where is this celery grown?
Peterman: Florida, upper Midwest, and California (primarily).
Steve Ela: Don’t the same problems with risk exist in conventional celery powder as they would organic?
Peterman: This relates to the scales of these industries. There are other uses for the celery powder in the conventional market (so more demand). Many companies do not only produce organic products, so the organic demand is enough to get their attention, but not enough to justify a new business element.
Comment from panel (unidentified): Conventional growers have been doing this for a long time; demand on organic is so low it needs to be an industry research project.
Dr. Andrew Milkowski (University of Wisconsin-Madison) states non-organic celery powder is still essential—no organic products contain high enough concentrations of nitrate or nitrite for curing meat. 80-200ppm of nitrite concentration are required to cure processed meat safely (based on meat weight).
Dr. Milkowski presented slides on the chemical processes involved in meat curing using this material [nitrate= celery powder; nitrite=fermented celery powder]. These highlighted issues included development of cured meat color (when the compound interacts with the meat), cured meat flavor, and celery powder’s functions as an antioxidant and antimicrobial.
He explains there are other vegetables and tubers that have enough nitrate in them to be candidates, but they have flavors and colors which limit their practical use (celeriac, Chinese cabbage, etc.).
Regarding human health risks from nitrate and nitrites, the International Agency for Research on Cancer (IARC) classifies processed meats (may or may not be cured) as group 1 carcinogens (consider them to be a cancer hazard). The IARC report noted that cured meats were a minor source of nitrate/nitrites and the source does not matter in this case. Dr. Milkowski states that modern knowledge suggests it’s not likely to be the nitrate/nitrites causing this increased risk.
Dr. Milkowski reviews nitrogen oxide physiology. The same compound that changes the color on cured meat has profound philological effects—it’s involved in blood pressure control, wound healing, etc. Nitrogen oxide in physiology is synthesized in the body, adjusted by dietary sources, recycled/excreted through saliva, and most exposure to nitrate/nitrite is entirely natural (largest exposure is vegetables).
Salivary glands naturally accumulate nitrate from the blood plasma. Bacteria in mouth likely have some enzyme activity, forming nitrite, which is then swallowed again (this is how it’s recycled). This process is likely beneficial to inhibiting microbes in the stomach, and Dr. Milkowski proposes this is a form of human innate immunity.
NOSB member Harriet Behar: Why is it that nitrate in water is so hurtful to pregnant women and babies (e.g. blue baby)?
Dr. Milkowski: Newborn infants have high residual content of fetal hemoglobin in their system (binds oxygen more strongly than oxygen in blood of mother). After birth that is replaced by normal hemoglobin; fetal hemoglobin is more sensitive to this compound. The intestinal system of newborns is virtually sterile, but as it gets populated, it gradually convert nitrates. This makes them more sensitive through the age of 6 months. The toxic dose for nitrite in adult humans is very, very high.
NOSB member Dan Seitz: Are there other methods used to cure meats? What about traditionally?
Dr. Milkowski: Preserving meat by salting is traditional. Saltpeter was very effective in preserving; saltpeter is sodium nitrate. In societies where food was scarce, that was an issue. In the 1920s, we learned that nitrate gets converted to nitrite (more efficient and we can use less of it).
NOSB member (unidentified): Comments on nitrates/nitrites in meat products indicate that we should compare it to drinking water requirements. Is that comparison relevant?
Dr. Milkowski: That’s a safety requirement for water that is consumed in large volumes. It’s not directly comparable. Those levels are regulated through USDA for ground products. For whole-muscle product, 200ppm are allowed (exception of bacon—must use 120ppm of sodium nitrate/nitrite and vitamin C or other compounds. When bacon is fried, you have the risk of forming nitrosamines).
NOSB member (unidentified): If we were to remove this from the National List or recommend its removal, how would it affect you in the work and research toward alternatives discussed?
Panel member(s) (unidentified): The industry would immediately destabilize development of new meat products. There would be high variability in how companies reacted; a lot of anxiety. In terms of accelerating/decelerating work, I don’t think it would have much effect. This is a mixed bag because you might motivate some but some might just not stick it out, whcih may reduce demand for the product and make it harder to bring out.
To be able to collect data we need to wait on the crop, and there is no way to accelerate the crop. Initial funding has gotten us a lot further ahead. Not sure how to make this a more critical issue to spur this on more. It would involve trying to explain to the grower that they need to take on the risk of growing an unknown plant. How do you get everyone to accept the risk on every level?
NOSB member Steve Ela: Where does the health data fall (comparing cancer risk studies and evidence humans can recycle nitrates)?
Panel member (unidentified): This is more of a sociological question. There are many studies using nitrates/nitrites ongoing (gives examples of medical uses/studies, such as Viagra). Sensitive teeth toothpaste has high levels.
The panel concludes and the NOSB breaks for lunch.
11:23 AM PT: Wasieleski: Explained steps in the celery powder production cycle. The ultimate goal is to get organic celery growers into the cycle. Celery acreage needs to be close to a juicing facility of an appropriate capacity because of short shelf-life and the risk of losing nitrate levels.
It is critical that there is an outlet for celery that doesn’t have the needed nitrate characteristics, in order to reduce risk to organic growers.
High nitrate levels can be achieved by strategically timing harvest.
Organic celery has shown inconsistent performance. It is a complex issue and future research with adequate funding is needed to address it.
11:12 AM PT: Dr. Silva continued. Her research looked at harvest dates and literature to understand what influences nitrate concentration in vegetables. Most research looks at keeping nitrate concentration low. They know that light influences nitrate concentrations. More research needs to be done regarding the best management within each variety.
On-farm sampling in the Madison, Wisconsin area indicated some environmental influence. Dr. Silva noted huge variability in nitrate levels depending on crop location which researchers are trying to understand.
They are seeking USDA Organic Research and Extension funding for a larger project.
NOSB member Emily Oakley: Did you look at varying levels of rainfall?
Dr. Silva: This could be a primary driver. Also need to look at soil temperature, cloud cover, etc.
11:04 AM PT: NOSB member Asa Bradman (Environmentalists / Resource Conservationists) introduced the session on celery powder: Conventional celery powder is used for curing organic meats as an alternative to synthetic nitrates. It has been on the National List for over 10 years and is going under sunset review. We also want to consider extending the full range of organic sources.
Celery Powder Panel:
Dr. Erin Silva (University of Wisconsin-Madison)
Ms. Jennifer Wasieleski (Kerry Foods)
Mr. Logan Peterman (Organic Valley/CROPP Cooperative)
Dr. Andrew L. Milkowski (University of Wisconsin-Madison)
Dr. Silva: Discussed celery powder research and alternatives. She has worked in organic vegetable production for 15 years. She is not a nitrate expert, but looks at how to grow organic crops in different regions. She was in a 2015 working group organized under the Organic Trade Association (OTA) to find an organic alternative to celery powder.
They studied whether increased use of synthetic nitrogen fertilizers are required to produce source plants with enough nitrate for celery powder production, compared with growing celery for vegetable production. The working group did not get a definitive answer to this question.
Grants awarded since 2015 funded a comprehensive survey. They looked at several variables that influence the amount of nitrate in the product, fertility management strategies, farm environment, and yellow and white beets (among others).
Dr. Silva described the research and findings. They found differences in nitrate amounts depending on variety, which offers opportunity for optimization. They found a great degree of variability year to year without altering production practices.
10:29 AM PT: Adam Cline, USDA National Agricultural Statistics Service (NASS), discusses USDA’s organic surveys and data quality.
The 2019 Census of Agriculture Special Study is currently posted in the Federal Register—comments close April 29. Data collection begins December 2019, results released in October 2020.
NASS conducts producer surveys and certifier surveys to get livestock and acreage data. The NOP has taken over the work of data collection from NASS and ERS. Data reports began in 2017 and NOP has provided multiple reports since then.
Reporting is currently voluntary, but there is a proposed rule to make this reporting mandatory. Voluntary reporting is increasing.
Cline explained the differences in NASS and NOP reporting
- NASS producer surveys: self-reported; certified crop and livestock.
- AMS NOP/Organic Integrity data: data mandatory, regulated reporting; AMS may count multiple operations where NASS would count those same operations as one operation. AMS also gets information from handlers (not just producers). Data issues (such as duplication) cannot be corrected. Record keeping varies greatly among certifiers. Currently these reports are less reliable but have potential.
Mandatory reporting will mean more data and better data. The NASS/NOP data validation project will also help improve data quality.
Cline notes the organic producer is more likely to be younger and more likely to be a full time farmer than non-organic farmers (64% of organic farmers report this).
NOSB member Steve Ela: Is income per farm up?
Cline: It appears to have gone up, but that data point is skewed because it does not consider the size of the farm. In the end it increased per-farm, but the increase is probably not drastic per-farm.
NOSB member Steve Ela: Any information on gross income per farm?
Cline: No, we only have it for the overall farm sector, not just the organic industry.
NOSB member Dave Mortensen: Is there any way of linking data you’re collecting based on acreages in transition—can you probe whether farms are increasing in size? Is it a function of production type? These kinds of things would help inform the NOSB (for example, are dairies getting much larger, etc.).
Cline: We could do this with overall data for all farms, but the organic census is very limited. With the 2019 survey coming up, we try and ask questions about what their future plans are.
NOSB member Dave Mortensen: And this data is available to the public?
Cline: Yes. At https://www.nass.usda.gov/AgCensus/
NOSB member Rick Greenwood (Environmentalists / Resource Conservationists): Can you do production by particular commodity per-acre? (Example: people say organic is less productive per-acre.)
Cline: You can compare it a little. ERS has tried to do some organic vs. conventional research. But it wouldn’t be from the 2017 survey (not broken out by organic).
NOSB member Harriet Behar: Been struggling about economic impact of organic. We know farm-gate prices, but there is gap in middle with understanding what the multiplier effects are regarding the economic impact of organic. Any way to distill these statistics to understand if an organic farm is generating more ripple effects through local communities than a conventional farm?
Cline: Not now, but we could add a set of questions for that.
10:08 AM PT: NOSB member Scott Rice (USDA Accredited Certifying Agent): Great to see that all the dairies were meeting 30% DMI requirement. Can you tell us whether that is true taking into account temporary confinement?
Tucker: Correct, 120 days and 30% was met taking into account allowed confinement.
(NOSB member, unidentified): Thanks for the origin of livestock (OOL) update. We have heard from dairy farmers that they need this rule now, so we encourage the NOP to not slow the process. They need this rule out there ASAP.
Tucker: If industry has changed a lot, it increases the need for public comment. If the community can articulate why the comments at the time are still relevant, those are the kinds of arguments that will have more weight. This decision will be made by USDA and Office of Management and Budget.
(NOSB member, unidentified): Who should those comments be directed to?
Tucker: We are the ones writing the work plan, and that’s where those comments are articulated. Send letters to me and Paul Lewis.
NOSB member Steve Ela (Organic Producer): Regarding dairy inspections: was that a cross section of regions and sizes?
Tucker: We used a risk-based approach, including compliance history. Hovering around 30% DMI is immediate trigger, etc.
NOSB member Tom Chapman (Handlers / Processors): Please continue to advertise and inform about enforcement activities.
Tucker: We have heard we need to do a better job of communicating how we do enforcement. The majority of our enforcement is against non-certified farms (representing themselves as organic falsely). We have improved reporting on website. Database improvements will make it easier to report to the public. Due process means we can’t post everything.
Emily Oakley: Consumers understand the three-year transition period. We hear that you need investigation, but it can be clearly asserted what the rules state. You raised issue of land—when the law was written, there was not an expectation that buildings would be erected. I hope you can issue clarity on this immediately.
9:55 AM PT: Tucker announced the Organic Integrity Data Quality award winners, based on data quality and quantity at the start of 2019. Examples of data quality include regular updates, use of product taxonomy, including acreage and livestock counts, and offering complete information.
Winners:
California Certified Organic Farmers (CCOF)
Colorado Dept. of Agriculture (CDA)
County of Marin Organic Certified Agriculture
Georgia Crop Improvement Assn. (GCIA)
Global Organic Alliance (GOA)
New Mexico Dept. of Ag. (NMDA)
One-cert, Inc. (ONE)
Primus Labs
[Cornucopia has been critical of the policies some of the certifiers in this list. See our Certifier Report for more.]
NOSB member Dan Seitz (Consumer / Public Interest Advocate): As you do fact finding and explore what is happening in the field, if you discover novel aspects to hydroponics that can’t be covered by the regulations, do you see yourself coming to the NOSB and working to develop additional rules that will address novel aspects of hydroponic operations?
Tucker: There are legal and policy questions. Growth of hydroponics/containers has raised policy questions. USDA disagrees that soil regulations mean all organic production must be soil-based. Instead they just apply to soil-based operations. There are the natural resources of the operation which is an important separate component of regulations. If there are areas the rules are being broken, that is a compliance problem and needs to be addressed with enforcement. If there are policy questions, we communicate that through the handbooks and through certifiers. If there are policy questions that need to be addressed by the NOSB, we will consider that. The vast majority of policy questions don’t need to come to the NOSB.
NOSB member Emily Oakley (Organic Producer): Can you clarify that all farm operations must adhere to sec 205.202 (the three-year transition/substance provision)?
Tucker: We are committed to upholding OFPA. There are questions about the boundaries of sec 205.202 and how certifiers should consider land-use history. We need to understand what is happening on the ground of these operations.
Oakley: Would a greenhouse that was previously conventional follow the three-year transition period? (Gives example about spraying prohibited substance on organic land and then setting up greenhouse on top of it.)
Tucker: Refuses to be involved in hypotheticals.
Oakley: Some of that can be clarified by following OFPA in every situation. Most people would think that can’t possibly be allowed. Can the NOP clarify the transition period ASAP?
Tucker: NOP will review the questions raised and will get it out to the world.
NOSB member Dave Mortensen (Scientist): When we voted on soil vs. hydroponic issue in Jacksonville, we did not define the suite of practices that would be acceptable. We’ve never had this discussion as NOSB. Given issues around plastics, metals, plastic, lack of vegetation, biodiversity, and more, he suggests the NOSB should discuss whether or not those practices conform to the organic label.
9:37 AM PT: Tucker also reported that a new rule is being drafted to strengthen organic enforcement, subject to interagency review, and she expects fall 2019 publication.
Strengthening Organic Enforcement:

Tucker then announced the launch of the Organic Integrity Learning Center (free online training for all professionals). Organic certifiers, inspectors, and reviewers are the target audience. Certifiers are responsible for getting staff registered. The public can make individual request for accounts.
9:32 AM PT: Tucker reported that all visits within the new Dairy Compliance Project have been unannounced, including single and multiple day visits. She considers the project to be highly successful. She reported learning a lot about training needs. She observed that the pasture rule is good but needs to be better enforced. [Cornucopia has talked in the past about the need for unannounced inspections and a lack of enforcement for the pasture rule for livestock.]
She saw challenges with how regional grazing season is defined, noting that certifiers need to look at regional grazing seasons and carefully evaluate breaks in grazing season. More training is needed with certifiers and operations, including more training on crop rotation.
Tucker reports that all operations they visited demonstrated at least 120 days of grazing, and all cows got 30% DMI during the season. Investigations are ongoing at operation and certifier level.
9:27 AM PT: The NOP is working with the USDA’s APHIS and U.S. Customs. In May, the NOP will send money to Customs to get import certificates in the system. They envision a global system.
The Farm Bill formalized the voting rule for the NOSB. The NOSB member nomination period is open with 5 vacancies, each with a 3-year term.
Regarding organic oversight of both import and domestic fraud, Tucker claimed that where investigations used to take years, they now take months.
Tucker reported the NOP recently completed farm-level review of three countries in the Black Sea Region. After looking at Organic System Plans (OSPs), reported yields are much higher than expected. The NOP is further investigating some farms reporting two to three times the average yield, including asking the certifiers of these operations how the yields are so high. The NOP is working with IOAS (previously International Organic Accreditation Service) to conduct two country commodity studies.
[Cornucopia’s white paper, The Turkish Infiltration of the U.S. Organic Market, exposed the issues in import fraud last year. Cornucopia continues to call on the USDA to take steps to enforce organic regulations at border crossings.]
The NOP is also continuing supply chain research, including use of dark web research techniques to find patterns of business relationships. The NOP is working with APHIS on fumigation investigations, and they are continuing ship-specific surveillance where there are sufficient details.
Tucker reports 180 operations (60%) in the Black Sea Region have lost certification, resulting in reduced organic grain and oilseed imports from Black Sea/Turkey.
Tucker also noted that a fumigated shipment of bell peppers into Philadelphia was relabeled (“reconditioned”) over the word “organic.” NOP wants to work with APHIS to continue this type of success.

9:13 AM PT: Tucker continues. NOP priorities include strong organic control systems, traceability, enforcement, support standards, and community collaboration.
Tucker shared program updates.
Origin of Livestock: AMS is exploring a second proposed Origin of Livestock Rule this fall, rather than a final rule. They continue to evaluate options but can reaffirm AMS is exploring moving ahead with this rule. (The interpretation of the origin of livestock rule has been abused by factory farm operations to allow access to cheap conventional cattle not raised organically.)
National List: Final rule published December 27 with 35 changes. Final rule is in clearance and they also have a proposed rule out. Sunset Material removal expedited this summer.
Compliance Database: Launched in March using increased funding. Doing a lot of enforcement work and can’t always talk about this in public.
Program Updates on Staffing: Bringing in Trade Systems Director from Customs and Border Protection. There are job announcements: hiring livestock compliance position and hiring auditors.
9:06 AM PT: NOP Administrator Tucker wants to clear the air regarding the recent glyphosate scandal. She says a group asked about a hypothetical scenario regarding hydroponic operations and she allowed herself to get drawn into a hypothetical. Her comments have been taken out of context and out of proportion. She emphasized she would evaluate everything case by case. This was not a program decision or policy statement. She has since learned who the certifier in question is and the NOP has engaged to find out what’s happening on the ground. Questions are currently being evaluated.
Glyphosate is not allowed in organic production. Certifiers are responsible for verifying compliance with site specific conditions. NOP investigates based on facts. We know some in organic community do not want hydroponics. Prior recommendation did not have enough detail to support a rule. NOP previously supported a task force and after a lot of work the community did not reach consensus. Hydroponics and container growing continue to be allowed. They are accountable to the same standards.
[Cornucopia’s report, Troubling Waters, explains more about the “organic” hydroponic issue. Cornucopia does not believe that hydroponic operations should be certified organic. Organic farming is based on careful nurturing of the soil, and hydroponic operations have no soil at all.]

8:58 AM PT: Behar: There are a lot of positives in organic: the market is growing and more farmers are using organic practices. However, she says, organic grain fraud from both foreign and domestic sources requires immediate enforcement.
The approval of hydroponic operations does not meet organic standards, and certifiers fear that clients might legally challenge certifier decisions has contributed to inconsistent standards.
Behar points out that various issues have been taken off the NOSB agenda. Whatever happened to inerts (pesticide ingredients); why don’t we have animal welfare standards; why aren’t we looking at hydroponics, BPA packaging, or pet food anymore? Commenters mentioned these and have expressed great frustration. NOP needs to address issues in a more timely way. We need regulations sooner rather than later.
Behar says the organic community is tenacious and we will work to solve these problems. Behar commends Tucker for communicating with community. Need to be more proactive before problems become the norm. Need to pursue consistency in high standards. We are an unusual agricultural sector; we want to be regulated.
An organic certificate represent commitment and good work, and consumers count on us. NOSB and NOP has responsibility to live up to consumer trust. “Without integrity, we are nothing.”
8:50 AM PT: Tucker turns meeting over to NOSB chair Harriet Behar (Environmentalist/Resource Conservationist seat).”
8:50 AM PT: Tucker turns meeting over to NOSB chair Harriet Behar (Environmentalist/Resource Conservationist seat).
Behar: Welcomes everyone to 55th NOSB meeting.
Steve Fuller, Washington State Department of Agriculture, welcomes everyone. He says the rich organic tradition in Washington is traced to farmer driven movement. Gives history of Washington state’s organic certification, which certifies over 1,300 operations. Agriculture has challenges: average farm revenue is down. On average farmers are getting older and having trouble attracting young farmers and losing land to climate change and urbanization. Need to get farmers a better economic return, and maybe a new generation will come to farming.
Organic must mean something. The soul of organics is at stake (quotes Michael Sligh)
Integrity, fairness, and transparency are key.
Will be disagreements over the next few days, but with integrity, fairness, and transparency you will define organic, he concludes.
8:32 AM PT: Deputy Administrator Tucker welcomes and officially opens the meeting.
Introduces USDA Team in the room: Paul Lewis, Theresa Mathews, Devin Patillo.
Tucker introduces David Glasgow, new Associate Deputy Administrator, who has been with the USDA over 15 years. Adam Kline from NASS is an expert on organic surveys.
Tucker thanks the NOSB. There are currently 14 members and they are recruiting 5 new members. The call for nominations is open and closes May 20. Even with 14 members, 10 votes are still needed to send a recommendation to NOP.

