Is carrageenan bad for you? When considering the answer to that question, it’s useful to follow the money.
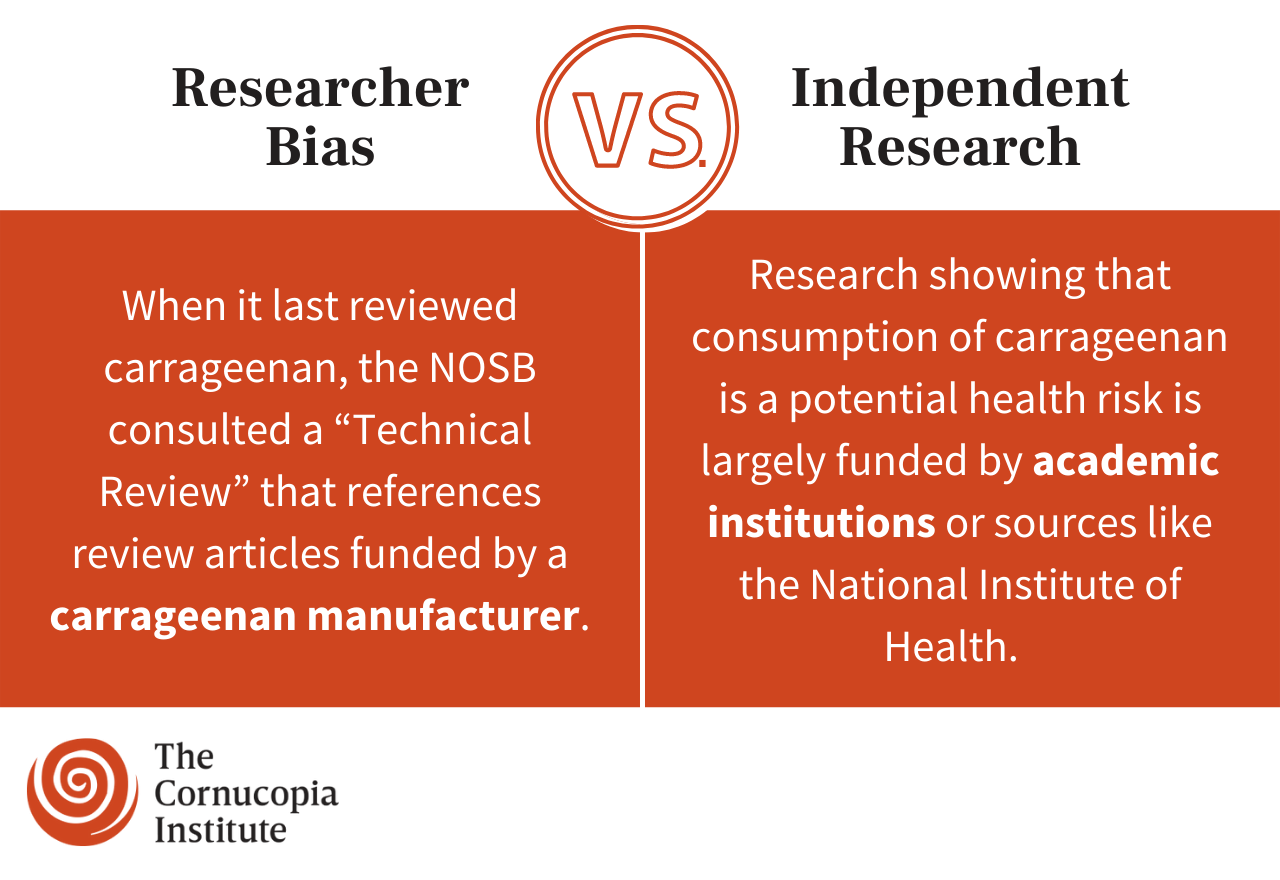
For example, industry consultant TOXpertise, LLC has painted research pointing to the potential health risks of this controversial food additive as faulty science. However, the company’s analyses were funded by FMC Corporation, which has “over 60 years of experience in the development and production of carrageenan products…”
Yet in a letter published as early as 1980 in The Lancet, one of the world’s leading medical journal, scientists expressed their concern with the safety of carrageenan in food.
Those scientists are in good company. Research showing that consumption of carrageenan is a health risk is typically conducted by academic institutions and funded through those institutions or sources like the National Institute of Health. On the other hand, if you investigate the source of studies insisting that carrageenan is safe for human consumption, you’ll find that they are often conducted or funded by the carrageenan industry or industry consultants.
This type of “researcher bias” is rampant in safety testing for food and products, when a researcher is often funded or employed by the industry producing that product. Outcomes can be influenced by study design choices, including who is chosen to include in a study and how data is interpreted.
The Cornucopia Institute believes the discussion concerning carrageenan must take into account the evolving research, the institutions behind that research, and increasing awareness and concern of consumers in the marketplace. An increasingly common presence in the typical Western diet, carrageenan deserves scrutiny. (Learn more about the use of carrageenan in organic food.)
Summary of Research
The following is a summary of the research, research funding, and affiliations of researchers and commenters. While not comprehensive, these studies were those found to be most applicable to the question of human health implications (because carrageenan is used to induce inflammation in countless animal experiments, this curated research sample focuses only on research into carrageenan itself) and whether carrageenan is a synthetic substance based on current manufacture techniques. The more recent research (since 2017) extends to actual clinical trials and research into carrageenan’s effect on actual human populations, not cell-line or animal experiments.
Note that this research review focuses only on the potential concerns about human health. There are thousands of research studies in which carrageenan is used to provoke biological responses in laboratory animals – those are not included here (but should certainly be considered in any future Technical Reviews).
1970s
Pittman K, Golberg L, and Coulston F. (1976) “Carrageenan: The effect of molecular weight and polymer type on its uptake, excretion and degradation in animals.” Food and Cosmetics Toxicology 14 (2):85-93.
Summary of findings: Food-grade carrageenan was given to guinea pigs, monkeys, and rats through drinking water or in the diet. Fecal and liver samples were examined by gel electrophoresis and carrageenans present in the feces were reduced to 100kDa or less. Carrageenans were also found in the liver, demonstrating that high molecular weight carrageenans are degraded after passing through the digestive tract and can be absorbed.
Author affiliations: Institute of Comparative and Human Toxicology, Center of Experimental Pathology and Toxicology, Albany Medical College (Albany, New York).
Engster M and Abraham R. (1976) “Cecal response to different molecular weights and types of carrageenan in the guinea pig.” Toxicology and Applied Pharmacology 38:265–282.
Summary of findings: In this short-term study, researchers administered different types of carrageenan in the diet and drinking water of guinea pigs for two weeks. They found ulceration of the intestines in guinea pigs given undegraded iota-carrageenan in the drinking water. No changes were observed in the other groups, and it is unclear what effects would have been seen if the experiment had been continued for longer than two weeks.
Funding: National Institute of Environmental Health Sciences, National Institutes of Health.
Author affiliation: Albany Medical College (Albany, New York).
Watanabe K, Reddy BS, Wong CQ, Weisburger JH (1978) “Effect of dietary undegraded carrageenan on colon carcinogenesis in F344 rats treated with azoxymethane or methylnitrosourea.” Cancer Research 38:4427–4430.
Summary of findings: This study found higher rates of tumors in rats fed undegraded carrageenan in the diet.
Funding: National Cancer Institute (National Institutes of Health).
Author affiliations: Naylor Dana Institute for Disease Prevention, American Health Foundation.
1980s
Watt J and Marcus R (1980) “Potential hazards of carrageenan.” The Lancet 315(8168): 602-603.
Letter to The Lancet: Leading carrageenan researchers R. Marcus and James Watt publish two letters in The Lancet, titled “Danger of Carrageenan in Foods” and “Potential Hazards of Carrageenan,” pointing out health concerns with the consumption of carrageenan. Highly respected, The Lancet is one of the world’s leading medical journals.
Watt J and Marcus R (1981) “Harmful effects of carrageenan fed to animals.” Cancer Detection and Prevention 4(1-4): 129-34.
Review article: The authors reviewed the scientific literature and found “an increased number of reports … describing harmful effects of degraded and undegraded carrageenan supplied to several animal species in their diet or drinking fluid.” “Harmful effects [of food-grade carrageenan] are almost certainly associated with its degradation during passage through the gastrointestinal tract. There is need for extreme caution in the use of carrageenan or carrageenan-like products as food additives in our diet.”
Watt J and Marcus R. (1981) “Danger of carrageenan in foods and slimming recipes.” The Lancet 317(8215): 338.
Letter to The Lancet: Scientists repeat their concern with the use of carrageenan in food in a letter to The Lancet.
Thomson AW & Fowler EF. (1981) “Carrageenan: a review of its effects on the immune system.” Agents and Actions, 11:265–273. https://link.springer.com/article/10.1007/BF01967625
Summary of findings: Carrageenans (kappa, lambda and iota) can markedly suppress immune responses both in vivo and in vitro. The mechanism responsible for carrageenan-induced immune suppression is believed to be its selective cytopathic effect on macrophages. This property of carrageenan has led to its adoption as a tool for analyzing the role of these cells in the induction and expression of immune reactivity.
Author affiliations: Department of Pathology, University of Aberdeen (Aberdeen, Scotland, UK) and the Department of Histopathology, St Bartholomew’s Hospital (London, UK).
Arakawe S, Okumua M, Yamada S, Ito M, Tejima S. (1986) “Enhancing effect of carrageenan on the induction of rat colonic tumors by 1,2-dimethylhydrazine and its relation to ß-glucuronidase activities in feces and other tissues.” Journal of Nutritional Science and Vitaminology 32:481–485.
Summary of findings: This study found higher rates of tumors in rats fed undegraded carrageenan in the diet.
Author affiliations: Nagoya City University (Nagoya, Japan).
Nicklin S and Miller K (1984) “Effect of orally administered food-grade carrageenans on antibody-mediated and cell-mediated immunity in the inbred rat.” Food and Chemical Toxicology 22:615–621.
Summary of findings: Researchers using undegraded carrageenan administered in the drinking water of rats showed that carrageenan penetrates the intestinal barrier.
Author affiliations: The British Industrial Biological Research Association, a privately-owned consulting firm.
Calvert RJ and Reicks M (1988) “Alterations in colonic thymidine kinase enzyme activity induced by consumption of various dietary fibers.” Proceedings of the Society for Experimental Biology and Medicine 189:45–51.
Summary of findings: Researchers examined the reported effects of various dietary fibers on chemically induced colon carcinogenesis in rats. This study found a four-fold increase in thymidine kinase activity (a measure for malignant disease) in colonic mucosa following exposure to food-grade carrageenan. No differences were found following exposure to guar gum (a food additive often used as an alternative to carrageenan).
Funding: Food and Drug Administration.
Author affiliations: Food and Drug Administration.
1990s
Weiner M. January, 1991. “Toxicological properties of carrageenan.” Agents and Actions, 32(1-2): 46-51. https://pubmed.ncbi.nlm.nih.gov/2058470/
Summary of findings: Author concludes that carrageenan is safe for human consumption based on the author’s review of various animal feeding studies (NOTE: there were few studies about carrageenan’s effect on human health at the time this study was published and this review omitted studies showing physiological effects, some of which are noted above).
Author affiliation: FMC Corporation (a chemical corporation and leading carrageenan manufacturer).
Wilcox DK, Higgins J, Bertram TA. September, 1992. “Colonic epithelial cell proliferation in a rat model of nongenotoxin-induced colonic neoplasia.” Laboratory Investigation, 67:405–411. https://pubmed.ncbi.nlm.nih.gov/1357233/
Summary of findings: This study shows an association between loss of epithelial cells (the cell membranes in the intestine) and the consumption of both undegraded and degraded carrageenan.
Funding: Proctor & Gamble Company.
Author affiliations: Proctor & Gamble Company.
Capron I, Yvon M, Muller G. April, 1996. “In-vitro gastric stability of carrageenan.” Food Hydrocolloids, 10(2):293–244. https://www.sciencedirect.com/science/article/abs/pii/S0268005X96800403
Summary of findings: This study analyzed the rate of degradation in an artificial stomach which simulated realistic conditions for human digestion, wherein the pH gradually decreases from 5 to 1.5 over 3 hours prior to gastric emptying. The findings showed that, under the most unfavorable conditions of gastric digestion (slow emptying rate and rapid acidification), about 10” of the carrageenan had a molecular weight of less than 100 kDa.
Funding: Proctor & Gamble Company.
Author affiliations: Proctor & Gamble Company.
Corpet DE, Taché S, and Préclaire M. March 19, 1997. “Carrageenan given as a jelly does not initiate, but promotes the growth of aberrant crypt foci in the rat colon.” Cancer Letters, 114:53–55. https://pubmed.ncbi.nlm.nih.gov/9103253/
Summary of findings: Consumption of food-grade carrageenan promotes the growth of aberrant crypt foci in the rat colon. Aberrant crypt foci are abnormal glands in the colon that are precursors to polyps and are one of the earliest changes seen in the colon that may lead to cancer.
Author affiliations: French National Institute of Agronomic Research (Toulouse, France).
Tobacman JK. July, 1997. “Filament disassembly and loss of mammary myoepithelial cells after exposure to lambda-carrageenan.” Cancer Research 57:2823-2826. https://pubmed.ncbi.nlm.nih.gov/9230181/
Summary of findings: Mammary myoepithelial cells exposed to lambda-carrageenan at rates as low as 0.00014% exhibited disruption of the internal cellular architecture and cell death. Destruction of these cells in tissue culture by a low concentration of a widely used food additive suggests a dietary mechanism for mammary carcinogenesis not considered previously.
Author affiliations: Department of Internal Medicine, College of Medicine, The University of Iowa (Iowa City, Iowa).
2000s
Suzuki J, Na HK, Upham BL, Chang CC and Trosko JE. 2000. “Lambda-carrageenan-induced inhibition of gap-junctional intercellular communication in rat liver epithelial cells.” Nutrition and Cancer, 36(1): 122-8. https://pubmed.ncbi.nlm.nih.gov/10798224/
Summary of findings: This study aimed to better understand the role of food-grade carrageenan in carcinogenesis. The experiments in this study were designed to test the hypothesis that carrageenan might function as a tumor-promoting chemical by inhibiting GJIC (Gap-junctional intercellular communication is believed to help healthy cells fight cancer). The data revealed inhibition of GJIC by carrageenan similar to that by the well-documented tumor promoter phorbol ester.
Author affiliations: Department of Pediatrics and Human Development, Michigan State University (East Lansing, Michigan).
Tobacman JK, Walters KS. 2001. “Carrageenan-induced inclusions in mammary myoepithelial cells.” Cancer Detect Prev., 25(6):520-6. PMID: 12132872. https://pubmed.ncbi.nlm.nih.gov/12132872/
Summary of findings: The purpose of this investigation was to characterize the ultrastructural changes that occur in mammary myoepithelial cells (MMEC) following exposure in tissue culture to low concentrations of lambda-carrageenan, a sulfated polysaccharide commonly used as a food additive. Carrageenan appeared to enter the cells by membrane-associated endocytic vesicles and accumulate in endosomes and lysosomes. Unusual lamellar inclusions were identified within lysosomes of the MMEC, and lysosomal vacuolation arose in association with the inclusions. The observed changes appeared to lead to destruction of the MMEC by release of proteolytic enzymes from the distorted lysosomes, similar to the process observed in lysosomal storage diseases.
Author affiliations: Department of Internal Medicine, University of Iowa (Iowa City, USA).
Tobacman JK. October 2001. “Review of Harmful Gastrointestinal Effects of Carrageenan in Animal Experiments.” Environmental Health Perspectives 109(10): 983-994. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1242073/
Review summary: This study examined existing research done to date (2001). The author concluded: “Because of the acknowledged carcinogenic properties of degraded carrageenan in animal models, and the cancer-promoting effects of undegraded carrageenan in experimental models, the widespread use of carrageenan in the Western diet should be reconsidered.”
Author affiliation: University of Iowa, College of Medicine, The University of Iowa (Iowa City, Iowa).
Hagiwara A, Miyashita K, Nakanishi T, Sano M, Tamano S, Asai I, Nakamura M, Imaida K, Ito N and Shirai T. 2001. “Lack of Tumor Promoting Effects of Carrageenan on 1,2-Dimethylhydrazine-induced Colorectal Carcinogenesis in Male F344 Rats.” Journal of Toxicologic Pathology 14; 37. https://www.jstage.jst.go.jp/article/tox/14/1/14_1_37/_article
Summary of findings: This study found no statistically significant increases in malignant tumors in rats given food-grade carrageenan in the diet. The study was terminated as higher rates of tumors in the carrageenan group were detected. The rats were killed after 90 days (a rat’s natural lifespan is 2 years). When the study was terminated, tumor rates were higher, but not yet high enough to be “statistically significant.”
Author affiliations: Nagoya City University, Daiyukai Institute for Medical Science and San-Ei Gen FFI, Inc. One of the authors has a work history with SanEi Gen FFI, Inc., a Japanese carrageenan manufacturer.
Uno Y, Omoto T, Goto Y, Asai I, Nakamura M and Maitani T. September, 2001. “Molecular weight distribution of carrageenans studied by a combined gel permeation/inductively coupled plasma (GPC/ICP) method.” Food Additives and Contaminants 18: 763-772. https://pubmed.ncbi.nlm.nih.gov/11552743/
Summary of findings: The study measured the molecular weight of 29 samples of food-grade carrageenan and concluded that no sample had a significant level of degraded carrageenan. The detection limit was 5%. However, the lowest average molecular weight detected over the three days was 718 kDa, indicating that some degradation of the carrageenan did occur
Author affiliations: San-Ei Gen FFI, Inc, a Japanese food additive manufacturer. In addition to carrageenan, San-Ei Gen FFI manufactures flavors, colors, preservatives and the artificial sweetener sucralose.
Cohen SM and Ito N. September, 2002. “A critical review of the toxicological effects of carrageenan and processed euchema seaweed on the gastrointestinal tract.” Critical Reviews in Toxicology, 32(5): 413-44. https://pubmed.ncbi.nlm.nih.gov/12389870/
Summary of findings: The authors of this review criticized research studies pointing to gastrointestinal harm from consuming carrageenan. The authors conclude that “there is no credible evidence supporting a carcinogenic effect or a tumor-promoting effect on the colon in rodents.”
Author affiliations: Department of Pathology/Microbiology, University of Nebraska Medical Center (Omaha, Nebraska) and Nagoya City University Medical School (Japan). The authors have ties to the carrageenan industry.
Weiner M, Nuber D, Blakemore WR, Harriman JF and Cohen SM. Epub September 7, 2006. “A 90-day dietary study on kappa-carrageenan with emphasis on the gastrointestinal tract.” Food and Chemical Toxicology, 45(1): 98-106. https://pubmed.ncbi.nlm.nih.gov/17034924/
Summary of findings: The study found no clinical signs in rats fed high doses of food-grade carrageenan with up to 12% degraded carrageenan, other than soft stool. The authors reported that the gastrointestinal tract “appeared normal” even in the rats given high doses of carrageenan in the diet.
Funding: FMC Corporation (a leading carrageenan manufacturer).
Author affiliations: FMC Corporation. In addition to manufacturing carrageenan, FMC Corporation produces pesticides and industrial chemicals.
Borthakur A, Bhattacharyya S, Dudeja PK and Tobacman JK. March, 2007. “Carrageenan induces interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells.” American Journal of Physiology, Gastrointestinal and Liver Physiology, 292(3): G829-38. https://pubmed.ncbi.nlm.nih.gov/17095757/
Summary of findings: Exposure of human colonic epithelial cells in tissue culture to small quantities of undegraded (food-grade) carrageenan produced inflammation by a second pathway of reactive oxygen species, as well as by the innate immune pathway.
Funding: Department of Veterans Affairs; National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Author affiliations: University of Illinois and Jesse Brown Veterans Affairs Medical Center (Chicago, Illinois).
Bhattacharyya S, Borthakus A, Dudeja PK and Tobacman JK. Epub April 12, 2007. “Carrageenan reduces bone morphogenetic protein-4 (BMP4) and activates the Wnt/beta-catenin pathway in normal human colonocytes.” Digestive Diseases and Sciences, 52(10): 2766-74. https://pubmed.ncbi.nlm.nih.gov/17429723/
Summary of findings: This study identified mechanisms by which food-grade carrageenan influences the development of human intestinal polyps. Untreated intestinal polyps can develop into colon cancer.
Funding: National Institutes of Health.
Author affiliations: University of Illinois at Chicago (Chicago, Illinois).
Tobacman JK, Bhattacharyya S, Borthakur A, Dudeja PK. 2008. “The carrageenan diet: not recommended.” Science, 321(5892):1040-1041. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5508865/
NOTE: This research was cited in the Handling Subcommittee summary review.
Summary of findings: Concluded that efforts at cultivation of carrageenan-producing seaweed have demonstrated how a “natural product” can produce harmful effects to the marine environment; similarly, harmful effects to humans may be attributable to carrageenan exposure.
Author affiliations: Department of Medicine, University of Illinois (Chicago, Illinois).
Bhattacharyya S, Dudeja PK and Tobacman JK, Epub April 11, 2008. “Carrageenan-induced NFkappaB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10.” Biochimica and Biophysica Acta., 1780(7-8): 973-82. https://pubmed.ncbi.nlm.nih.gov/18452717/
Summary of findings: Exposure to human colonic epithelial cells in tissue culture to small quantities of food-grade carrageenan produced inflammatory responses.
Funding: National Institutes of Health.
Author affiliations: University of Illinois (Chicago, Illinois).
Bhattacharyya S, Borthakur A, Dudeja PK and Tobacman JK. March, 2008. “Carrageenan induces cell cycle arrest in human intestinal epithelial cells in vitro.” Journal of Nutrition, 138(3): 469-75. https://pubmed.ncbi.nlm.nih.gov/18287351/
Summary of findings: Exposure of human colonic epithelial cells in tissue culture to small quantities of undegraded (food-grade) carrageenan produced an increase in cell death with cell cycle arrest, effects that can contribute to ulcerations.
Funding: National Institutes of Health.
Author affiliations: University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center (Chicago, Illinois).
Bhattacharyya S, Gill R, Chen ML, Zhang F, Linhardt RJ, Dudeja PK and Tobacman JK. April 18, 2008. “Toll-like receptor 4 mediates induction of the Bcl10-NFkappaB-interleukin-8 inflammatory pathway by carrageenanin human intestinal epithelial cells.” Journal of Biological Chemistry, 283(16): 10550-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2447641/
Summary of findings: Exposure of human colonic epithelial cells in tissue culture to small quantities of food-grade carrageenan was associated with changes in molecular signaling pathways that resemble the changes found in human colonic polyps. Untreated polyps can develop into colon cancer.
Funding: National Institutes of Health; Veterans Administration.
Author affiliations: University of Illinois (Chicago, Illinois); Jesse Brown Veterans Affairs Medical Center (Chicago, Illinois); and Rensselaer Polytechnic Institute (Troy, New York).
Bhattacharyya S, Borthakur A, Tyagi S, Gill R, Chen ML, Dudeja PK, Tobacman JK. January, 2010. “B-cell CLL/lymphoma 10 (BCL10) is required for NF-kappaB production by both canonical and noncanonical pathways and for NF-kappaB-inducing kinase (NIK) phosphorylation.” Journal of Biological Chemistry. 1;285(1):522-30. https://pubmed.ncbi.nlm.nih.gov/19897484/
Summary of findings: Carrageenan stimulates innate immune-mediated pathways of inflammation.
Funding: National Institutes of Health; Veterans Administration
Author affiliations: University of Illinois (Chicago, Illinois).
Bhattacharyya S, Liu H, Zhang F, Jam M, Dudeja PK, Michel G, Linhardt RJ, and Tobacman JK. October, 2010. “Carrageenan-induced innate immune response is modified by enzymes that hydrolyze distinct galactosidic bonds.” Journal of Nutritional Biochemistry 21(10): 906-13. https://pubmed.ncbi.nlm.nih.gov/19864123/
Summary of findings: This study examines the immune response by which food-grade carrageenan causes inflammation. Researcher findings indicate an upstream signaling role for BCL10, in addition to its effects on IKKgamma, the regulatory component of the IKK signalosome, and a requirement for BCL10 in both canonical and noncanonical pathways of NF-kappaB activation. Essentially, the commonly used food additive carrageenan can be added to the short list of known activators of both immune pathways.
Funding: Veterans Administration.
Author affiliations: University of Illinois (Chicago, Illinois); Jesse Brown Veterans Affairs Medical Center (Chicago, Illinois); Rensselaer Polytechnic Institute (Troy, New York); University Pierre and Marie Currie/Sorbonne University (Paris, France).
Bhattacharyya S, Dudeja PK and Tobacman JK. Epub October 11, 2010. “Tumor necrosis factor alpha-induced inflammation is increased but apoptosis is inhibited by common food additive carrageenan.” Journal of Biological Chemistry 285(50): 39511-22. https://pubmed.ncbi.nlm.nih.gov/20937806/
Summary of findings: This study examines the particular mechanisms by which food-grade carrageenan cause inflammation. Researchers found that common food additive carrageenan (CGN) stimulates inflammation through both the canonical and non-canonical pathways of NF-κB activation and utilizes the adaptor molecule BCL10 (B-cell leukemia/lymphoma 10).
Funding: Veterans Administration.
Author affiliations: University of Illinois (Chicago, Illinois) and Jesse Brown Veterans Affairs Medical Center (Chicago, Illinois).
Bhattacharyya S, O-Sullivan I, Katyal S, Unterman T and Tobacman JK. January, 2012. “Exposure to the common food additive carrageenan leads to glucose intolerance, insulin resistance and inhibition of insulin signaling in HepG2 cells and C57BL/6J mice.” Diabetologia 55(1): 194-203. https://pubmed.ncbi.nlm.nih.gov/22011715/
Summary of findings: The results of this initial research suggest that carrageenan in the diet may contribute to developing diabetes. This is the first report of the impact of carrageenan on glucose tolerance and indicates that carrageenan impairs glucose tolerance, increases insulin resistance and inhibits insulin signaling in vivo in mouse liver and human HepG2 cells. These effects may result from carrageenan-induced inflammation. The results demonstrate extra-colonic manifestations of ingested carrageenan and suggest that carrageenan in the human diet may contribute to the development of diabetes.
Funding: National Institutes of Health and American Diabetes Association.
Author affiliations: University of Illinois (Chicago, Illinois).
Yang B, Bhattacharyya S, Linhardt R and Tobacman JK. Epub March 5, 2012. “Exposure to common food additive carrageenan leads to reduced sulfatase activity and increase in sulfated glycosaminoglycans in human epithelial cells.” Biochimie 94(6): 1309-16. https://pubmed.ncbi.nlm.nih.gov/22410212/
Summary of findings: Exposure to small amounts of food-grade carrageenan reduces the activity of sulfatase enzymes, which are critical for many vital cellular processes.
Funding: National Institute of General Medical Sciences, National Institutes of Health.
Author affiliations: University of Illinois (Chicago, Illinois); Jesse Brown Veterans Affairs Medical Center (Chicago, Illinois); Rensselaer Polytechnic Institute (Troy, New York).
Borthakur A, Bhattacharyya S, Anbazhagan AN, Kumar A, Dudeja PK and Tobacman JK. Epub May 8, 2012. “Prolongation of carrageenan-induced inflammation in human colonic epithelial cells by activation of an NFκB-BCL10 loop.” Biochimica and Biophysica Acta 1822(8): 1300-7. https://pubmed.ncbi.nlm.nih.gov/22579587/
Summary of findings: Inflammation of the colon caused by exposure to low levels of food-grade carrageenan persists beyond the initial period of exposure.
Funding: National Institutes of Health.
Author affiliations: University of Illinois (Chicago, Illinois).
Bhattacharyya S, Feferman L, Borthakur S and Tobacman JK. Epub December 13, 2013. “Common Food Additive Carrageenan Stimulates Wnt/β-Catenin Signaling in Colonic Epithelium by Inhibition of Nucleoredoxin Reduction.” Nutrition and Cancer 66(1): 117-127. https://pubmed.ncbi.nlm.nih.gov/24328990/
Summary of findings: Exposure to carrageenan may be a risk factor in the development of colorectal cancer. The findings indicate that environmental exposure stimulates both Wnt signaling and suggest that carrageenan exposure in vivo may contribute to development of colonic neoplasia (uncontrolled growth of cells). Average daily intake of carrageenan in the U.S. in the 1970s was calculated to be 108 mg by the National Academy of Sciences, but recently the average daily carrageenan intake was reported to be ∼250 mg/day. Increased attention to the effects of carrageenan on vital cell processes, including the Wnt/β-catenin pathway, may lead to significant clinical benefit, as well as increased understanding of relationships between environmental exposures and human disease.
Funding: Veterans Affairs Merit Award.
Author affiliations: University of Illinois (Chicago, Illinois) and Jesse Brown Veterans Affairs Medical Center (Chicago, Illinois).
Bhattacharyya S, Feferman L, and Tobacman JK. Epub April 28, 2014. “Increased Expression of Colonic Wnt9A through Sp1-mediated Transcriptional Effects involving Arylsulfatase B, Chondroitin 4-Sulfate, and Galectin-3.” The Journal of Biological Chemistry, 289(25): 17564-17575. https://pubmed.ncbi.nlm.nih.gov/24778176/
Summary of findings: Mechanism by which Wnt expression was increased by carrageenan exposure was unknown. This study showed that Sp1 activated Wnt9A transcription through changes in arylsulfatase B, chondroitin 4-sulfation, and galectin-3. In conclusion, a decline in arylsulfatase B leads to transcriptional effects mediated by Sp1 and galectin-3. The significance is that extracellular events can regulate transcription through changes in arylsulfatase B and chondroitin 4-sulfation.
Author affiliations: University of Illinois (Chicago, Illinois) and Jesse Brown Veterans Affairs Medical Center (Chicago, Illinois).
Bhattacharyya S, Feferman L, and Tobacman JK. December 12, 2014. “Regulation of Chondroitin-4-Sulfotransferase (CHST11) Expression by Opposing Effects of Arylsulfatase Bon BMP4 and Wnt9A.” Biochim Biophys Acta 1849(3): 342-352. https://pubmed.ncbi.nlm.nih.gov/25511584/
Summary of findings: Exposure to the common food additive carrageenan, which reduces ARSB activity, reduced expression of bone morphogenetic protein (BMP)-4 in colonic epithelium and increased Wnt9A expression and Wnt/β-catenin signaling.
Funding: University of Illinois (Chicago, Illinois).
Author affiliations: Department of Medicine, University of Illinois (Chicago, Illinois).
Jung TW, Lee SY, Hong HC, Choi HY, Yoo JH, Baik SH, and Choi KM. January 25, 2014. “AMPK activator-mediated inhibition of endoplasmic reticulum stress ameliorates carrageenan-induced insulin resistance through the suppression of selenoprotein P in HepG2 hepatocytes.” Molecular and Cellular Endocrinology 382(1):66-73. https://pubmed.ncbi.nlm.nih.gov/24055274/
Summary of findings: Carrageenan causes inflammation through toll-like receptor 4, which plays an important role in insulin resistance and type 2 diabetes mellitus. Carrageenan induces endoplasmic reticulum (ER) stress in a time- and dose-dependent manner. ER stress plays a crucial role in selenoprotein P regulation. Salsalate relieves ER stress and is a new therapeutic strategy to treat insulin resistance.
Author affiliations: Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Guro Hospital.
Bhatacharyya S, Feferman L, Unterman T, and Tobacman JK. March 25, 2015. “Exposure to common food additive carrageenan alone leads to fasting hyperglycemia and in combination with high fat diet exacerbates glucose intolerance and hyperlipidemia without effect on weight.” Journal of Diabetes Research Volume 2015, Article ID 513429. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4390184/
Summary of findings: Mice exposed to 10mg/L food grade lambda and kappa carrageenan in drinking water and fed an 8% fat diet for 1 year showed glucose intolerance after 6 days and earlier onset of fasting hyperglycemia, higher glucose levels, and exacerbated dyslipidemia compared with the control. This suggests that carrageenan exposure may exacerbate harmful effects of a high fat diet and contribute to development of diabetes.
Author affiliations: Department of Medicine, University of Illinois (Chicago, Illinois).
Weiner M. L., Ferguson H. E., Thorsrud B. A., et al. March, 2015. “An infant formula toxicity and toxicokinetic feeding study on carrageenan in preweaning piglets with special attention to the immune system and gastrointestinal tract.” Food and Chemical Toxicology. 2015;77:120–131. doi: 10.1016/j.fct.2014.12.022. https://pubmed.ncbi.nlm.nih.gov/25592784/
Summary of findings: This study looked at the short-term (28 day) effect of different concentrations of carrageenan on piglets. Note that while they conclude there was no ill effect, glucosuria was observed in the animal models (the presence of reducing sugars in the urine). This research was not looking at glucose metabolism or insulin resistance, and the observed glucosuria is relevant considering other studies into carrageenans metabolic effects.
Author affiliations: The authors of this research paper are from the carrageenan industry: TOXpertise, LLC, Abbott Nutrition, (Columbus, OH); MPI Research, (Mattawan, MI), and Celtic Colloids Inc., (Topsham, ME).
Bhattacharyya S, Feferman L, and Tobacman JK. March 25, 2015. “Carrageenan inhibits insulin signaling through GRB10-mediated Decrease in Tyr(p)- ISR1 and through Inflammation-induced Increase in Ser(P)307-IRS1.” Journal of Biological Chemistry 290(17): 10764-10774. https://pubmed.ncbi.nlm.nih.gov/25883986/
Summary of findings: Inflammation induced by exposure to the common food additive carrageenan leads to insulin resistance by increase in Ser(P) (307)-insulin receptor substrate 1 (IRS1) and subsequent decline in the insulin-stimulated increase in Ser(P)(473)-AKT. Studies were performed in human HepG2 cells and in C57BL/6J mice. and indicate that carrageenan inhibited insulin signaling by two mechanisms. These mechanisms provide internal feedback, mediated by Ser(P)(473)-AKT, Ser(P)(401)- GATA2, and nuclear GATA2, which modulates insulin responsiveness.
Author affiliations: University of Illinois (Chicago, Illinois) and Jesse Brown Veterans Affairs Medical Center (Chicago, Illinois).
Tobacman JK. October 27, 2015. “The Common Food Additive Carrageenan and the alpha-gal epitope.” Journal of Allergy and Clinical Immunology 136(6): 1708-9. https://pubmed.ncbi.nlm.nih.gov/26518095/
Summary of findings: Antibodies to the oligosaccharide epitope galactose-a-1,3- galactose (alpha-gal) are of considerable interest because they are so prevalent, include all isotypes, and are specific to humans and Old World apes. Alpha-gal–mediated responses, including immediate and delayed anaphylaxis, appear to be increasing. In the recent review ‘‘The alpha-gal story: lessons learned from connecting the dots,’’ sources of exposure to the alpha-gal epitope were presented, with particular attention to cetuximab, mammalian meat products, and tick bites. This communication is intended to bring attention to including carrageenan, a very commonly used food additive, to the list of sources of exposure to the alpha-gal epitope.
Author affiliations: Department of Medicine, University of Illinois (Chicago, Illinois).
Coleman MT. December 22, 2015. “Dairy-free” dietary substitute, abdominal pain, and weight loss.” Clinical Medical Reviews and Case Reports 2:8. https://clinmedjournals.org/articles/cmrcr/clinical-medical-reviews-and-case-reports-cmrcr-2-079.pdf
Summary of findings: Elimination of carrageenan containing almond milk from the diet of a patient that had substituted it for cow’s milk several months prior resulted in stabilization of weight and resolution of abdominal pain. Certain food substitutions for dairy products may expose patients to additives like carrageenan, for which there is evidence of its contribution to gastrointestinal disturbances. Considering an etiology for gastrointestinal symptoms brought on by dietary additives in the diagnostic differential gives the practitioner avenues to pursue prior to ordering expensive testing and treatments.
Author affiliations: Louisiana State University School of Medicine (New Orleans, Louisiana).
Weiner, M. January, 2016. “Parameters and pitfalls to consider in the conduct of food additive research, Carrageenan as a case study.” Food and Chemical Toxicology, 87: 31-44. https://pubmed.ncbi.nlm.nih.gov/26615870/
Summary of findings: FMC Corporation announced that this analysis found significant weaknesses in food additive research, using carrageenan as a primary example.
Funding: FMC Corporation, an agricultural sciences company and stakeholder in the carrageenan industry.
Author affiliations: Dr. Myra L. Weiner, is the owner and president of TOXpertise, LLC, a firm that consults for the carrageenan industry.
Munyaka PM, Sepehri S, Ghia J-E, Khafipour E. April 5, 2016. “Carrageenan gum and adherent invasive Escherichia coli in a piglet model of inflammatory bowel disease: impact on intestinal mucosa-associated microbiota.” Front Microbiol 2016;7:462. https://www.frontiersin.org/articles/10.3389/fmicb.2016.00462/full
Summary of findings: Research into inflammatory bowel disease (in piglets) found that carrageenan-induced colitis decreased bacterial species richness and shifted microbial community composition. Researchers demonstrated that carrageenan resulted in bacterial dysbiosis and shifted community composition, similar to what has been previously observed in human IBD patients.
Funding: Since 2001, the federal government has invested almost $159 million to help support indirect costs of research at the University of Manitoba.
Author affiliations: Department of Immunology, University of Manitoba, (Winnipeg,Canada); Department of Animal Science, University of Manitoba, (Winnipeg, Canada); Children Hospital Research Institute of Manitoba (Winnipeg, Canada); Section of Gastroenterology, Department of Internal Medicine (Winnipeg, Canada); Inflammatory Bowel Disease Clinical & Research Centre, University of Manitoba (Winnipeg, Canada); Department of Medical Microbiology, University of Manitoba (Winnipeg, Canada).
Weiner M, McKim J, and Blakemore W. “Addendum to Weiner, M.L. 2016. “Parameters and Pitfalls to Consider in the Conduct of Food Additive Research, Carrageenan as a Case Study.” Food Chemical Toxicology 87, 31-44. https://pubmed.ncbi.nlm.nih.gov/28651808/
Summary: This paper is an addendum to a 2016 paper outlining pitfalls and parameters to consider in the conduct of food additive research with carrageenan.
Author affiliations: TOXpertise, LLC; IONTOX, LLC; and Celtic Colloids, Inc. All of these are associated with the carrageenan industry.
Tavassoli-Kafrani E, Shekarchizadeh H, Masoudpour-Behabadi M. February 10, 2016. “Development of edible films and coatings from alginates and carrageenans.” Carbohydrate Polymers, 137: 360-374. https://www.sciencedirect.com/science/article/abs/pii/S0144861715010541
Summary of review: This review highlights production and characteristics of carrageenan and alginate as sources of film-forming materials. Water-soluble hydrocolloids like polysaccharides usually impart better mechanical properties to edible films and coatings than do hydrophobic substances. They also are excellent barriers to oxygen and carbon dioxide making them useful in extending shelf lives.
Author affiliations: Department of Food Science and Technology, College of Agriculture, Isfahan University of Technology (Isfahan, Iran).
McKim Jr JM, Heidi Baas, Rice G, Willoughby Sr J, Weiner M, Blakemore W. October, 2016. “Effects of carrageenan on cell permeability, cytotoxicity, and cytokine gene expression in human intestinal and hepatic cell lines.” Food and Chemical Toxicology, 96: 1-10. https://www.sciencedirect.com/science/article/pii/S0278691516302265
Summary of findings: Three common forms of the food additive carrageenan were tested in vitro cell lines, looking for permeability, cytotoxicity, and induction of cytokines. Carrageenan was negative in all endpoints evaluated.
Author affiliations: All the authors hail from businesses and consultants associated with carrageenan manufactures. The specific affiliations include: IONTOX, LLC; Cyprotex; TOXpertise, LLC; Celtic Colloids, Inc. Dr. Myra L. Weiner, is the owner and president of TOXpertise, LLC, a firm that consults for the carrageenan industry.
SINCE 2017
Sumit Bhattacharyya, et al. March 31, 2017. “A Randomized Trial of the Effects of the No-carrageenan Diet on Ulcerative Colitis Disease Activity.” 181 – 192. DOI: 10.3233/NHA-170023. https://content.iospress.com/articles/nutrition-and-healthy-aging/nha170023
Summary of findings: A randomized, double-blind, placebo-controlled, multicenter, clinical trial published in 2017 showed that people with colitis should avoid carrageenan. Patients who received carrageenan-containing capsules relapsed, and none of the patients who received placebo-containing capsules relapsed in their colitis disease. Laboratory tests showed increases in inflammatory biomarkers in those trial participants who received carrageenan.
Author affiliations: Department of Medicine, University of Illinois (Chicago, Illinois); Jesse Brown VA Medical Center (Chicago, Illinois); Department of Medicine, University of Chicago, (Chicago, Illinois); Division of Epidemiology and Biostatistics, University of Illinois at Chicago, (Chicago, Illinois) and Faculty of Health Sciences, Simon Fraser University (Burnaby, BC, Canada); Department of Kinesiology and Nutrition, University of Illinois at Chicago, (Chicago, Illinois).
Bixler, HJ. April 5, 2017. “The carrageenan controversy.” Journal of Applied Phycology, 29:2201–2207. https://link.springer.com/article/10.1007/s10811-017-1132-4
NOTE: This paper was cited in the subcommittee summary review.
Summary of article: (NOTE this is not a research paper) The article describes how the negative attitude toward carrageenan evolved, stating it came from research from a group at the University of Illinois who claim that carrageenan upregulates inflammatory genes of the intestinal epithelium. There is no evidence that this in vitro model applies in vivo and a growing body of research is showing it does not. Nevertheless, it was picked up by various bloggers feeding on contradictory issues and quickly went viral. This paper describes the evolution of the “carrageenan controversy” and provides information for food producers and consumers on new more robust studies confirming that it is safe to consume foods containing carrageenan. This article also presents actions being taken by carrageenan producers and users, to reduce the noise in the public domain from the controversy.
Author affiliations: Ingredients Solutions, Inc, a carrageenan supplier. The company calls themselves “The World’s Largest Independent Supplier of Carrageenan” and is a world leader in the development, design and marketing of Specialty Hydrocolloids.
John Vincent Martino, Johan Van Limbergen1, and Leah E. Cahill. May 1, 2017. “The Role of Carrageenan and Carboxymethylcellulose in the Development of Intestinal Inflammation.” Front. Pediatr., 1(5):96. doi:10.3389/fped.2017.00096. https://pubmed.ncbi.nlm.nih.gov/28507982/
Summary of findings: This review found that even non-degraded carrageenan can cause inflammation and bowel disorders, suggesting that food-grade carrageenan may contribute to ulcers and IBD. Research found that carrageenan may trigger or magnify an inflammatory response in the human intestine. The researchers determined that more study was needed because it seemed consumption of carrageenan was a notable risk factor but did not seem to be the sole cause involved in the development of IBD or in disease recurrence after treatment. The researchers also highlighted the prevalence of carrageenan in pediatric diets as a cause for concern.
Author affiliations: Pediatric Gastroenterology, Hepatology and Nutrition, IWK Health Centre, (Halifax, NS, Canada); Medicine, Dalhousie University (Halifax, NS, Canada); and Department of Nutrition at the Harvard T.H. Chan School of Public Health (Boston, Massachusetts).
Younes M, Aggett P, Aguilar F, et al. April 26, 2018. “Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives.” EFSA J; 16:e05238. https://doi.org/10.2903/j.efsa.2018.5238.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7009739/
Summary of article: This opinion deals with the re-evaluation of the safety of food-grade carrageenan and processes Eucheuma seaweed and these substances use as food additives in the European Union. Taking into account the lack of adequate data to address the uncertainties surrounding the chemistry, exposure assessment, and biological and toxicological data., the Panel concluded that the acceptable daily intake (ADI) for carrageenan and processed Eucheuma seaweed of 75 mg/kg bw per day should be considered temporary.[Note that US-Americans are predicted to eat 250mg of food-grade carrageenan daily.]
Author affiliations: European Food Safety Authority (EFSA).
Demeng Zhang, Mengxue Zhang, and Xiaoxiao Gu. 2018. “8 – Seaweed-Derived Hydrocolloids as Food Coating and Encapsulation Agents.” Bioactive Seaweeds for Food Applications, Academic Press, Pages 153-175. https://www.sciencedirect.com/science/article/pii/B978012813312500008X
Summary of chapter: (NOTE this is an excerpt from the book Bioactive Seaweeds for Food Applications, Natural Ingredients for Healthy Diets). This chapter discusses the food applications of carrageenan (and the types of carrageenan) including its uses as a edible film coating or general films on food products.
Author affiliations: Qingdao BrightMoon Seaweed Group (Qingdao, China). Bright moon seaweed group belongs to the seaweed industry and develops and sells products of seaweed. They own six manufacturing sectors including alginate, functional sugar alcohol, Marine cosmetics, Marine functional food, Marine biological medical materials, Marine biological fertilizer, and “Marine lifestyle” health chain service sectors.
David Shlomit, et al. Epub February 22, 2018. “Revisiting the carrageenan controversy: do we really understand the digestive fate and safety of carrageenan in our foods?” Food Funct., 10, 1763. DOI: 10.1039/C9FO00018F. https://pubmed.ncbi.nlm.nih.gov/29469913/
Summary of findings: A review of carrageenan safety research in 2019 came to three conclusions. First, they concluded that there isn’t enough information about current consumption rates. Second, the link between carrageenan’s properties, its impact on digestion, and the colon microbiome and inflammation are yet to be fully resolved. Third, there is not enough research on carrageenan’s effect on predisposed populations, such as elderly people or IBD patients. The review of the existing concluded carrageenan has not been definitively determined as “safe” and more research needs to be done.
Author affiliations: Laboratory of Chemistry of Foods and Bioactives, Department of Biotechnology and Food Engineering, Technion, Israel Institute of Technology (Haifa, Israel).
James M. McKim, Jamin A. Willoughby Sr., William R. Blakemore, & Myra L. Weiner. November 16, 2018. “Clarifying the confusion between poligeenan, degraded carrageenan, and carrageenan: A review of the chemistry, nomenclature, and in vivo toxicology by the oral route.”
Critical Reviews in Food Science and Nutrition, 59:19, 3054-3073, DOI: 10.1080/10408398.2018.1481822 https://www.tandfonline.com/doi/full/10.1080/10408398.2018.1481822
Summary: This piece is a review of existing research, discussing the parameters for what carrageenan is in comparison to other substances. This review is critical of research conducted that shows potential harm from food-grade carrageenan and the presence of poligeenan in commercial applications. Note: some of the review’s statements of fact are not backed up with citations.
Funding: This review was funded by FMC Corporation (a chemical corporation and leading carrageenan manufacturer).
Author Affiliations: Apart from the association with FMC Corporation, the authors have affiliations with TOXpertise, LLC and IONTOX, LLC. Both TOXpertise and IONTOX provide professional consulting services in the field of toxicology, and are known consultants for the carrageenan industry. One author is affiliated with and Celtic Colloids, Inc., a consulting company that provides technical assistance to the carrageenan industry.
Myra L. Weiner and James M. McKim, Jr. February 22, 2019. “Comment on ‘Revisiting the carrageenan controversy: do we really understand the digestive fate and safety of carrageenan in our foods?’ by S. David, C. S. Levi, L. Fahoum, Y. Ungar, E. G. Meyron-Holtz, A. Shpigelman and U. Lesmes, Food Funct., 9: 1344–1352. https://pubs.rsc.org/en/content/articlelanding/2019/fo/c8fo01282b
Summary: This piece is a comment rather than research or review. The comment is critical of the above paper.
Author Affiliations: TOXpertise, LLC and IONTOX, LLC. Both TOXpertise and IONTOX provide professional consulting services in the field of toxicology, and both businesses are known to be hired as consultants for the carrageenan industry. Note the previous “reviews” in this listing, including the same author (Weiner, M), that were critical of carrageenan research done through non-industry sources.
Bhattacharyya S., Feferman L., Tobacman J. K. April 15, 2019. “Distinct Effects of carrageenan and high-fat consumption on the mechanisms of insulin resistance in nonobese and obese models of type 2 diabetes.” Journal of Diabetes Research; 2019:14. doi: 10.1155/2019/9582714.9582714. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6501429/
Summary: In this research, carrageenan increased serum galectin-3 and increased binding of galectin-3 with the insulin receptor in the carrageenan-induced mouse model of nonobese diabetes. Essentially, exposure to low concentration of food-grade carrageenan (10 mg/L) for only six days led to glucose intolerance and insulin resistance in a mouse model.
Author Affiliations: Department of Medicine, University of Illinois at Chicago and Jesse Brown VA Medical Center, (Chicago, IL).
Ye Mi Y, et al. March, 2020. “Native κ-carrageenan induced-colitis is related to host intestinal microecology.” International Journal of Biological Macromolecules, 147: 284-294. DOI: 10.1016/j.ijbiomac.2020.01.072. https://www.sciencedirect.com/science/article/abs/pii/S0141813019390828
Summary of findings: Research into inflammation and carrageenan in 2020 found that inflammatory properties of carrageenan are related to carrageenan’s modification of the intestinal microbiome. In addition, researchers found that carrageenan can exacerbate chronic inflammation (which could explain why people with existing chronic conditions improve with a carrageenan-free diet).
Author affiliations: Human Health Research Laboratory, College of Food Science and Engineering, Ocean University of China (Qingdao, China); Institute of Ocean and Earth Sciences, University of Malaya (Kuala Lumpur, Malaysia).
Leo Feferman, et al. April 21, 2020. “Carrageenan-Free Diet Shows Improved Glucose Tolerance and Insulin Signaling in Prediabetes: A Randomized, Pilot Clinical Trial.” J Diabetes Res. 2020: 8267980.doi: 10.1155/2020/8267980. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7191375/
Summary of findings: Due to previous research showing that low concentrations of carrageenan in drinking water caused marked glucose intolerance and insulin resistance in mice, this clinical study tested the impact of the no-carrageenan diet in prediabetes. The clinical study found significant differences between the no-carrageenan and carrageenan-in-diet groups, suggest that removing carrageenan may improve insulin signaling and glucose tolerance. This is a preliminary small trial requiring more research.
Author affiliations: Department of Medicine, College of Medicine, University of Illinois at Chicago and Jesse Brown VA Medical Center, (Chicago, IL); Department of Nutrition, College of Applied Health Sciences, University of Illinois at Chicago (Chicago, IL); and Department of Epidemiology and Biostatistics, College of Public Health, University of Illinois at Chicago (Chicago, IL).
Bhat, Mugdha & Sharma, Ashwani & n rao, Nagashree & Biotechnology, B. July, 2020. “Carrageenan-based edible biodegradable food packaging: A review.” Journal of Food Science and Nutrition. 5. 69-75. https://www.researchgate.net/publication/344016334_Carrageenan-based_edible_biodegradable_food_packaging_A_review
Summary of review: This review summarizes food packaging and edible food film uses for carrageenan. Edible coatings consist of a layer of the carrageenan polysaccharide that is sprayed, dipped, or spread over the surface of certain fruits and produce to extend shelf life.
Author affiliations: Rashtreeya Vidyalaya College of Engineering (Bengaluru, Karnataka, India).
Alireza Naseri, et al. August, 2020. “Multi-Extraction and Quality of Protein and Carrageenan from Commercial Spinosum (Eucheuma denticulatum).” Foods, 9(8):1072. doi: 10.3390/foods9081072. https://www.researchgate.net/publication/343565314_Multi-Extraction_and_Quality_of_Protein_and_Carrageenan_from_Commercial_Spinosum_Eucheuma_denticulatum
Summary of findings: This paper summarizes and evaluates carrageenan extraction methods.
Author affiliations: Research Group for Bioactives—Analysis and Application, The National Food Institute, Technical University of Denmark (DTU Food) (Lyngby, Denmark); Innovation, CP Kelco (Lille Skensved, Denmark). CP Kelco is a carrageenan manufacturer.
de Lima Barizão C, et al. December 15, 2020. “Biodegradable films based on commercial κ-carrageenan and cassava starch to achieve low production costs.” International Journal of Biological Macromolecules, 165 (Part A): 582-590. ISSN 0141-8130. https://www.sciencedirect.com/science/article/abs/pii/S0141813020345098
Summary of findings: Biodegradable films were produced by casting commercials kappa-carrageenan (κ-car) and cassava starch at different κ-carrageenan/cassava starch weight ratios. Physical, thermal, and mechanical properties were evaluated.
Author affiliations: Laboratory of Materials, Macromolecules, and Composites (LaMMAC), Federal University of Technology – Paraná (UTFPR), (Apucarana, Brazil); Analitycal Applied in Lipids, Sterols, and Antioxidants (APLE-A), State University of Maringá (UEM), Colombo avenue, (Maringá, Brazil); Group of Polymeric Materials and Composites (GMPC), Department of Chemistry, State University of Maringá (UEM), (Maringá, Brazil).
Zhou J, et al. March, 2021. “Long-term kappa-carrageenan consumption leads to moderate metabolic disorder by blocking insulin binding.” Pharmacological Research, 165: 105417. https://www.sciencedirect.com/science/article/abs/pii/S1043661820317254
Summary of findings: The purpose of this study was to investigate the impact of κ-carrageenan (CGN) on glucose intolerance and insulin resistance from the perspective that κ-CGN may interfere with insulin receptor function and affect insulin sensitivity and signaling, thereby leading to body weight loss. The study concluded that κ-CGN reduced weight gain without affecting food intake, but impaired glucose metabolism in mice by interfering with insulin binding to receptors, causing non-diabetic weight gain reduction due to metabolic disorder.
Author affiliations: State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-Products, Ningbo University, (Ningbo, Zhejiang, China); Department of Laboratory Medicine, Ningbo Medical Center Lihuili Hospital, (Ningbo, Zhejiang, China); Department of Laboratory Medicine, Taipei Medical University Ningbo Medical Center, (Ningbo, Zhejiang, China).
Fang Liu, et al. June 19, 2021. “Food-grade carrageenans and their implications in health and disease.” Comprehensive Reviews in Food Science and Food Safety 20(1). https://onlinelibrary.wiley.com/doi/full/10.1111/1541-4337.12790
Summary of findings: This research reviewed the molecular mechanisms by which carrageenans exert their biological effects and examined the interactions between carrageenans and the gut microbiome in the pathogenesis of gastrointestinal disorders. This review argues for personalized guidance on carrageenan intake based on individuals’ health status. Future research efforts that aim to close the knowledge gap on the effect of low-dose and chronic carrageenan intake as well as interactions among food additives should be conducive to the improved safety profile of carrageenans in processed food products.
Author affiliations: College of Food Science and Engineering, Ocean University of China (Qingdao, China); Affiliated Hospital of Qingdao Binhai University, Qingdao, China; Laboratory of Marine Drugs and Biological Products, Pilot National Laboratory for Marine Science and Technology (Qingdao, China); USDA-ARS Animal Genomics and Improvement Laboratory (Beltsville, Maryland).
Ali AJ, Abdulla HI, Al-Nimer MS. June 19, 2021. “The Pharmacological Effects of Kappa Carrageenan on Different Human Cell Lines and Genomic DNA: An in vitro study.” Iraqi J Pharm Sci, Vol 30:1. https://bijps.uobaghdad.edu.iq/index.php/bijps/article/view/1229
Summary of findings: Kappa-carrageenan inhibited the cancer cell growth and fibroblast cell lines growth (in vitro) experimental model. The carrageenan solution completely and significantly damaged the DNA molecule bin the research. This study shows that the k-carrageenan pharmaceutical preparations exert biological activities as anticancer in vitro studies. The authors reference how carrageenan’s properties lead it to be commonly used in the oral healthcare products and cosmetics.
Author affiliations: International Association for Dental Research (Alexandria, Virginia); Hawler Mediacl University (Erbil, Iraq).
Yeshi Yin, et al. September 20, 2021. “Carrageenan oligosaccharides and associated carrageenan-degrading bacteria induce intestinal inflammation in germ-free mice.” Journal of Genetics and Genomics, 48(9): 815-824. https://www.sciencedirect.com/science/article/abs/pii/S1673852721002459
Summary of findings: This research explores the so-far uninvestigated effects on host inflammatory response by carrageenan-degrading bacteria and the associated products carrageenan degradation. Researchers looked into carrageenan degradation by human fecal microbiota and the inflammatory response to carrageenans and/or carrageenan-degrading bacteria in germ free mice. The research found that high molecular weight carrageenan remained undegraded in the presence of human fecal microbiota, but low molecular weight κ-carrageenan was degraded when exposed to seven of eight human fecal samples. This study illustrated that the action of carrageenan on microflora can promote pro-inflammatory effects.
Author affiliations: State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products, Institute of Food Research ( Zhejiang Academy of Agricultural Sciences, China); Shandong Provincial Key Laboratory of Glycoscience and Glycotechnology, Key Laboratory of Marine Drugs of Ministry of Education (Ocean University of China, China); Department of Gastroenterology, Children’s Hospital (Zhejiang University School of Medicine, China); Department of Laboratory Animal Science, College of Basic Medical Sciences (Third Military Medical University, China); Department of Biomedical Sciences, Joan C. Edwards School of Medicine, (Marshall University, United States).
Barbara Borsani, et al. September 27, 2021. “The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand?” Nutrients, 13(10), 3402; https://doi.org/10.3390/nu13103402. https://www.mdpi.com/2072-6643/13/10/3402
Summary of findings: The authors address the debate on the safety of carrageenan in the Western Diet (addressing that carrageenan’s consumption has increased). This review aimed to discuss the role of carrageenan in inflammatory bowel diseases and allergic reactions, citing the existing evidence that carrageenan may pose a threat to human health. For example, the authors cite that carrageenan is extensively used as an inflammatory and adjuvant agent in vitro and in animal experimental models for the investigation of immune processes or to assess the activity of anti-inflammatory drugs. Carrageenan can also activate the innate immune pathways of inflammation, alter the gut microbiota composition and the thickness of the mucus barrier. Clinical evidence suggests that carrageenan is involved in the pathogenesis and clinical management of inflammatory bowel diseases (IBD), (further evidenced by the fact that food-exclusion diets can be an effective therapy for disease remission). Specific IgE to the oligosaccharide α-Gal found in carrageenan has been associated with allergic reactions commonly referred to as the “α-Gal syndrome”. The authors of the review conclude that gaps in scientific understanding of the health effect of carrageenan need to be filled and advise limiting human exposure to carrageenan.
Author affiliations: Department of Pediatrics, Vittore Buzzi Children’s Hospital, University of Milan (Milan, Italy).
Ivan Kushkevych and Josef Jampílek. December 2021. “Chapter 4 — Effect of intestinal microbiome, antibiotics, and probiotics in the prevention and management of ulcerative colitis.” Probiotics in the Prevention and Management of Human Diseases, A Scientific Perspective. 2022, Pages 59-92. https://www.sciencedirect.com/science/article/pii/B9780128237335000167
Summary of findings: This chapter briefly addresses the role of carrageenan in the development of ulcerative colitis.
Author affiliations: Department of Experimental Biology, Faculty of Science, Masaryk University, (Brno, Czech Republic); Department of Analytical Chemistry, Faculty of Natural Sciences, Comenius University (Bratislava, Slovakia); Institute of Neuroimmunology, Slovak Academy of Sciences (Bratislava, Slovakia).
Lara Hart, et al. December 21, 2021. “Nutritional Therapies and Their Influence on the Intestinal Microbiome in Pediatric Inflammatory Bowel Disease.” Nutrients 2022, 14, 4. https://pubmed.ncbi.nlm.nih.gov/35010879/
Summary of findings: This paper addresses the role of environmental risk factors, including carrageenan in the diet, in inflammatory bowel disease (IBD), Crohn’s disease (CD), and ulcerative colitis (UC). The intestinal microbiota is strongly influenced by diet. The association between the increasing incidence of IBD worldwide and increased consumption of a westernized diet suggests host nutrition may influence the progression or treatment of IBD via the microbiome.
Author affiliations: Department of Paediatrics, Division of Paediatric Gastroenterology & Nutrition, McMaster University (Hamilton, ON L8N 3Z5, Canada); McMaster Children’s Hospital ( Hamilton, Canada); Department of Pediatric Gastroenterology and Nutrition, Amsterdam University Medical Centers, Emma Children’s Hospital (Amsterdam, The Netherlands); Tytgat Institute for Liver and Intestinal Research, Amsterdam Gastroenterology Endocrinology and Metabolism, Amsterdam University Medical Centers, University of Amsterdam (Amsterdam, The Netherlands); Amsterdam Reproduction & Development Research Institute, Amsterdam University Medical Centers, Emma Children’s Hospital (Amsterdam, The Netherlands); Edmonton Paediatric IBD Clinic, Division of Paediatric Gastroenterology and Nutrition, Departments of Paediatrics & Physiology, University of Alberta (Edmonton, Canada); College of Medicine and Health, University College Cork (Cork, Ireland); Department of Paediatrics, Division of Paediatric Gastroenterology and Nutrition, Children’s Hospital of Los Angeles (Los Angeles, USA); Department of Paediatrics, Dalhousie University (Halifax, Canada); Farncombe Family Digestive Health Research Institute, McMaster University (Hamilton, Canada); Centre for Metabolism, Obesity and Diabetes Research, McMaster University, (Hamilton, Canada).
Oliver Sandys & Anje te Velde. January 4, 2022. “Raising the Alarm: Environmental Factors in the Onset and Maintenance of Chronic (Low-Grade) Inflammation in the Gastrointestinal Tract.” Digestive Diseases and Sciences. https://doi.org/10.1007/s10620-021-07327-1 https://link.springer.com/article/10.1007/s10620-021-07327-1
Summary of findings: This paper highlights the contribution of common environmental factors towards deterioration of GI health (including carrageenan in the diet) and the induction of pathophysiology associated with onset and maintenance of chronic inflammation in the GI tract.
Author affiliations: Amsterdam UMC, University of Amsterdam, Tytgat Institute for Liver and Intestinal Research, AGEM, (Amsterdam, The Netherlands); Vrije Universiteit Amsterdam (Amsterdam, The Netherlands).
Pauline Raoul, Marco Cintoni, Marta Palombaro, Luisa Basso, Emanuele Rinninella, Antonio Gasbarrini, and Maria Cristina Mele. January 13, 2022. “Food Additives, a Key Environmental Factor in the Development of IBD through Gut Dysbiosis.” Microorganisms; 10(1), 167; https://doi.org/10.3390/microorganisms10010167. https://www.mdpi.com/2076-2607/10/1/167/htm
Summary of findings: This article belongs to the Special Issue Gastrointestinal Microbiota and Gut Barrier Impact Human Health and Disease. This paper directly addresses the role carrageenan (and other additives) in IBD and gut dysbiosis, based on a comprehensive review of the current research available to them at the time. They noted that carrageenan clearly causes detrimental changes to the human gut microbiome, which can fuel various inflammatory proceses (including intesitinal permeability). They also disucss how all carrageenans increased levels of flagellin (measures of gut permeability) among other concerning health risks. The paper concludes by saying that evidence has been accumulating about the severe impact of the consumption carrageenan on gut microbiota and its positive associations with IBD and other inflammatory processes through gut microbial modulation.
Author affiliations: UOC di Nutrizione Clinica, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario A. Gemelli (Rome, Italy:
Dipartimento di Medicina e Chirurgia Traslazionale, Università Cattolica Del Sacro Cuore (Rome, Italy); and, UOC di Medicina Interna e Gastroenterologia, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario A. Gemelli (Rome, Italy)
Zilong Guo, et al. January, 2022. “Carrageenan oligosaccharides: A comprehensive review of preparation, isolation, purification, structure, biological activities and applications.” Algal Research, 61: 102593. https://doi.org/10.1016/j.algal.2021.102593. https://www.sciencedirect.com/science/article/abs/pii/S2211926421004124
Summary of findings: This review aimed to comprehensively summarize the methods of preparation, structural characteristics, and the biological activities of carrageenan oligosaccharides (which are the degradation products of carrageenan).
Author affiliations: College of Food Science and Light Industry, Nanjing Tech University, (Nanjing, China); Faculty of Fisheries Sciences, Hokkaido University, (Hokkaido, Japan).
Wei Wu, et al. February, 2022. “Dietary κ-carrageenan facilitates gut microbiota-mediated intestinal inflammation.” Carbohydrate Polymers, 277: 118830. https://www.sciencedirect.com/science/article/abs/pii/S0144861721012170
Summary of findings: This study examined the pathway through which carrageenan can have inflammatory effects. Research showed that food-grade carrageenan did cause reduced bacteria-derived short-chain fatty acids (SCFAs) and decreased thickness of the mucus layer by altering microbiota composition. When researchers administered pathogenic bacterium, it further aggravated the inflammation and mucosal damage in the presence of κ-carrageenan. These results suggest that food-grade κ-carrageenan creates an environment that favors inflammation by perturbation of gut microbiota composition and then facilitates expansion of pathogens.
Author affiliations: State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products, Ningbo University, Ningbo, China); Collaborative Innovation Center for Zhejiang Marine High-efficiency and Healthy Aquaculture, (Ningbo University, China); Department of Gynecology and Obstetrics, the Affiliated Hospital of Medical College of Ningbo University, (Ningbo, China); Department of Laboratory Medicine, Ningbo Medical Centre Lihuili Hospital, (Ningbo University, China).
Juanjuan Guo, et al. January 28, 2022. “Physicochemical dynamic changes and differences of κ-carrageenan in different vehicles (aqueous and casein solution) during in vitro gastrointestinal digestion.” Food Hydrocolloids, 107553. https://www.sciencedirect.com/science/article/abs/pii/S0268005X2200073X
Summary of findings: This research sought to understand the inflammatory mechanism of carrageenan in the gastrointestinal tract, the differences of κ-carrageenan (κ-CGN) in different vehicles (aqueous and casein solutions) and the physicochemical changes were investigated during in vitro digestion. Overall, the presence of casein reduced the exposure of sulfate groups and improved the conformational stability of κ-CGN. Therefore, the differences of the conformation and exposed sulfate group content of CGN in different vehicles might contribute to the different inflammatory effects in the gastrointestinal tract, which has guiding significance to the safe consumption of CGN.
Author affiliations: College of Oceanology and Food Sciences, Quanzhou Normal University (Quanzhou, Fujian, China); College of Food Science, Fujian Agriculture and Forestry University (Fuzhou, Fujian, China); Key Laboratory of Inshore Resources Biotechnology (Quanzhou Normal University) Fujian Province University, (Quanzhou, China).
Yuan Gao, et al. June 15, 2022. “The risk of carrageenan-induced colitis is exacerbated under high-sucrose/high-salt diet.” International Journal of Biological Macromolecules, Volume 210: 475-482. ISSN 0141-8130, https://doi.org/10.1016/j.ijbiomac.2022.04.158. https://www.sciencedirect.com/science/article/pii/S0141813022008728
Summary of findings: This study explored the risk of κ-carrageenan-induced colitis under high-sucrose or high-salt diet in mice. Findings were that the carrageenan under high-sucrose diet significantly reduced colon length and induced more serious deepening of the crypts. Intake of carrageenan under high-sucrose/high-salt diet induced more serious goblet cell reduction and increased intestinal permeability. Finally it was found that the intake of carrageenan under high-sucrose/high-salt diet significantly reduced the abundance of anti-inflammatory bacterium and increased the abundance of harmful bacterium, which was significantly related to the decrease of anti-inflammatory metabolites in colon. Overall, high-sucrose or high-salt diet increased the risk of carrageenan-induced colitis.
Author affiliations: Laboratory of Food Science and Human Health, College of Food Science and Engineering, Ocean University of China (Qingdao, Shandong, China); Laboratory of Marine Drugs and Biological Products, Pilot National Laboratory for Marine Science and Technology (Qingdao, Shandong, China); Collaborative Innovation Center of Seafood Deep Processing, Dalian Polytechnic University (Dalian, China).
Johnsrud, Celine; Tahiri, Mirlinda. 2022. “Effects of carrageenan and carboxymethylcellulose on intestinal health: a scoping review.” Norwegian University of Life Sciences. https://nmbu.brage.unit.no/nmbu-xmlui/handle/11250/3007212
Summary of findings: The cited scoping review (thesis) reviewed current scientific reviews of carrageenan. The review concluded that while more research should be done, the exisiting research (as of 2022) suggested that carrageenan can cause adverse effects on intestinal health concerning barrier function and/or permeability, including inflammation and intestinal microbiota.
Author affiliations: Norwegian University of Life Sciences
Roberta Imperatore, Graziella Orso, Serena Facchiano, Pierpaolo Scarano, Seyed Hossein Hoseinifar, Ghasem Ashouri, Carmine Guarino, Marina Paolucci. January 30, 2023
“Anti-inflammatory and immunostimulant effect of different timing-related administration of dietary polyphenols on intestinal inflammation in zebrafish, Danio rerio.” Aquaculture, Volume 563(1): 738878. https://doi.org/10.1016/j.aquaculture.2022.738878. https://www.sciencedirect.com/science/article/abs/pii/S0044848622009954
Summary of findings: This illustrates in part how that k-carrageenan is used frequently in animal-based experiments to cause inflammation for research purposes. The substance is specifically ued in modeling k-carrageenan-induced intestinal inflammation. Here, k-carrageenan was used to cause morphological alterations in zebra fish, such as the decrease of intestinal folds with consequent expansion of gut lumen, and increased number of goblet cells, as well as the over-regulation of pro-inflammatory markers and reduction of the antioxidant enzymes immunoexpression.
Author affiliations: Department of Sciences and Technologies, University of Sannio (Benevento, Italy); Department of Fisheries, Gorgan University of Agricultural Sciences and Natural Resources (Gorgan, Iran).

